Macaca arctoides (Geoffroy Saint-Hilaire, 1831)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6867065 |
|
DOI |
https://doi.org/10.5281/zenodo.6863173 |
|
persistent identifier |
https://treatment.plazi.org/id/CE199B17-FFD1-FFD5-FA36-60AAF930F8B9 |
|
treatment provided by |
Jonas |
|
scientific name |
Macaca arctoides |
| status |
|
18. View Plate 36: Cercopithecidae
Stump-tailed Macaque
French: Macaque brun / German: Barenmakak / Spanish: Macaco rabon
Other common names: Bear Macaque, Stumptail Macaque
Taxonomy. Macacus arctoides Geoffroy Saint-Hilaire, 1831 View in CoL ,
Cochinchine, a region in southern Vietnam; based on the mounted skin of an adult male collected by P. M. Diard in June 1822, for which the exact place of collection is unknown.
Morphological evidence (genital morphology, female sexual skin morphology, and facial skin color), copulatory behavior patterns, blood proteins, and distribution suggest that M. arctoides is derived from the sinica species group, more specifically from a M. thibetana-like ancestor. Some authors recognize subspecies based on pelage differences. Monotypic.
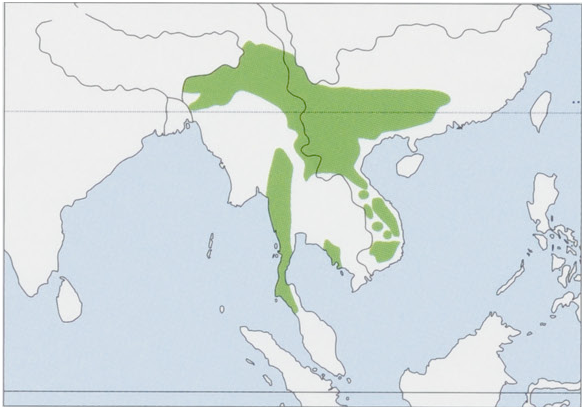
Distribution. S & SE Asia, NE India S and E of the Brahmaputra River (Assam, Arunachal Pradesh, Meghalaya, Nagaland, Manipur, Mizoram, and Tripura states), SW China S of 25° N (Yunnan, Guizhou, Guangxi, and Guangdong provinces), N Myanmar, Bangladesh, Thailand, Laos, Cambodia, and N Peninsular Malaysia where it could be a recent colonizer dispersed from S Thailand as recently as 1959; it may be now extinct in Bangladesh where it was last recorded in 1982 and 1989. Known records are, at present, concentrated along N and S margins ofits distribution, and it is rare or absent in the C Indochinese Peninsula, where deciduous forests predominate. The species has been introduced into Hong Kong. View Figure
Descriptive notes. Head-body 51:7-65 cm (males) and 48.5-53 cm (females), tail 1.7-8 cm (males) and 1.7-6 cm (females); weight 9.9-15.5 kg (males) and 7.5-9.1 kg (females). The Stump-tailed Macaque is a moderately large macaque. Pelage is long and silky, with hairs in the scapular region of adult males up to 115 mm long. Dorsal pelage color usually is brown, occasionally reddish (erythristic) or blackish (melanistic); individually variable sporadic traits may occur in the same group. Outer surfaces of limbs are about the same color as the trunk, or slightly paler. Pelage of the shorttail varies from well-furred to partly hairless. Fur on underparts is thin and slightly paler than on the dorsal surface but considerably darker than the visible ventral skin. Crown hairs radiate from the center and are long at the back and sides. Anterior crown hairs are short and brown, creating a bald appearance. Side whiskers and beard are slightly paler than dorsal pelage. Face, including cheekbone regions,is thinly haired. Skin on orbital and cheekbone regions is pink to red, and areas around the nose and mouth are variably buffy to blackish. Adult females are about the same color as adult males. In old age, scattered gray hairs appear in both sexes, and facial skin may lose its reddish color. Stump-tailed Macaques are cranially distinguished by broad anteriorly directed cheekbones (malars) and relatively small canines. Newborn pelage is whitish, and facial skin is pale pink. In older infants, dorsal pelage begins to darken from whitish to buffy to brown on the lower back; dorsal surface of the trunk is brown by about one year of age. Infants also are distinguished by a characteristically strong odor.
Habitat. Primary broadleaf-evergreen rainforest in hilly areas and adjacent degraded and disturbed habitats. Stump-tailed Macaques can also be found in mixed deciduous/bamboo forest. The known elevational range is 50-2800 m, and there are no records from extreme lowlands. Climate throughout the distribution of the Stumptailed Macaque varies from tropical to subtropical. Chronically low food availability may characterize some of these habitats. Apparently it is totally absent from offshore islands; it 1s not known to swim.
Food and Feeding. Diets of wild Stump-tailed Macaques contain mostly vegetable materials, including fruit, seeds, flowers, leaves, and roots. They dig tubers and roots out of the ground. Two hundred plants were identified as food sources of Stump-tailed Macaques during a field study in Jorhat District, Assam, India; seeds and fruits constituted more than 45% of the food intake. At least 18 species of cultivated plants are raided for food. Rice and especially corn were raided in an area of slash-and-burn agriculture formerly occupied by Hmong hill people at an elevation of ¢.1000 m in lower montane forest in western Thailand. Stump-tailed Macaques opportunistically eat animal food, including insects (adults and larvae), freshwater crabs, frogs, birds, and bird eggs.
Breeding. Reproductive anatomy of the Stump-tailed Macaqueis strikingly distinct in both sexes. The male’s glans penis is lanceolate and about twice as long as in other species of macaques, and the baculum (penis bone) is extremely elongated and straight or gently curved in form. Scrotum is red, and the penis is deep bluish-purple. The female’s prominent vaginal collicle, short vagina, and long exocervix (at least 50% longer than in any other macaque species) are morphologically complementary to the genitalia of the male. The vulvar area of adult females likewise is red, and the clitoris is dark bluish-purple. The male’s testes descend into the scrotum at about three years of age, and the onset of semen production may occur about a year later. Onset of menstruation in captive females occurs about two years of age, first ovulation before age four, and first birth before age five. Sexual maturity in captive males and females, and presumably in natural populations, occurs 2-3 years before full adult size is reached. Captive females do not exhibit cyclical swelling of sexual skin or other obvious changes in perineal morphology during various phases of a 30day ovarian cycle. Nevertheless, references to sexual skin swelling and receptive/proceptive behavior continue to occur in the literature. Copulatory behavior may be less dependent on female hormone levels than in other macaque species, but copulation frequency was observed to be variably elevated around mid-cycle in some colony groups. Copulation may be initiated by either sex. A female initiates copulation by presenting her hindquarters to a male. A male initiates copulation by approaching, touching, or threatening a female, thereby inducing her to present her hindquarters. A male responds to hindquarter presentation by visual, oral, olfactory, and digital examination of the female’s perineum, and subsequently mounts her dorsoventrally. Pelvic thrusting usually continues through a single uninterrupted mount until ejaculation occurs (single-mount ejaculation). In colony groups, numbers ofthrusts per ejaculation were 26-64 and took 36-59 seconds. A “frowning round-mouthed expression” is exhibited by males at ejaculation. Intromission usually continues while the male sits and pulls the female onto his lap (the unique “pairsit”), with mean duration of 67-114 seconds. Copulations in colony groups usually occur in short series that involve a single male-female pair and may be monopolized by competitive dominant males. Limited evidence suggests the possibility of seasonal breeding in natural populations. The mean gestation ofsingle young in 93 colony pregnancies was 177-3 days (extremes 161-210 days). Mean birth weight for infants in two colonies was 495 g. Infants in one laboratory colony were nursed for at least 7-9 months. Until a new infant is born, offspring may be accepted on the nipple even after lactation has ceased. The interbirth interval averages 1-6 years. In semi-natural groups, reproductive rate was estimated to be one infant per adult female every two years. Age of menopause is unknown, although reduction in reproductive capacity may occur before 17 years of age. Maximum known longevity in captivity is c.30 years.
Activity patterns. Stump-tailed Macaques wake at about dawn; feed until 10:00-11:00 h; rest, groom, and play during midday; feed again in the late afternoon beginning ¢.17:00 h; and retire to one or a few sleeping trees. Percentages of time allocated to different activities in a group in Jorhat District were 39-9% resting, 24-8% feeding, 16-9% traveling, 15-6% grooming, and 2-4% engaging in other activities. Stump-tailed Macaques usually forage and travel on the ground but are able to travel and forage in all levels of the forest canopy. Midday activities and feeding on seasonally available fruits, seeds, and leaves may take place in trees. In response to danger, they usually flee on the ground, but occasionally into the canopy. They can be aggressive in encounters with humans.
Movements, Home range and Social organization. Home ranges of Stump-tailed Macaquesin Jorhat District are 336-587 ha, with core areas of 193-320 ha. Daily movements of wild populations are 2-3 km in Yunnan, China, and 1-8-2-3 km in Assam, India; a semi-wild group in peninsular Thailand moved 0-4-3 km/day. Groups of Stump-tailed Macaques are multimale-multifemale. The ratio of adult males to adult females is c.1:6 in Tripura, India and 1:1-4 in Assam, India. Twenty adult males and 16 adult females were recorded in a free-ranging group of 73 individuals in southern Thailand. Group sizes vary from 2-3 individuals to ¢.60-70. A few solitary males have been reported in north-eastern India and western Thailand. It seemslikely that young males in wild populations leave their natal group at or before sexual maturity. There was one record of a group of 100 or more individuals in peninsular Thailand, and unverified verbal accounts of similar numbers elsewhere in peninsular Thailand and western Thailand in the 1970s—-1980s.
Status and Conservation. CITES Appendix II. Classified as Vulnerable on The IUCN Red List. The Stump-tailed Macaque appears to have been fairly common throughout much of its distribution as recently as the early 20" century, but it has become generally rare because of the burgeoning human population and rapidly expanding economic development. In South Asia,it is considered critically endangered and may have become extinct in Bangladesh. The population in north-eastern India may have been reduced to seven subpopulations totaling less than 250 individuals because its specialized habitat has been degraded and fragmented through selective logging, timber and firewood collection for charcoal, deliberately set fires, and the construction of dams, roads, and powerlines, with consequent soil loss and erosion. Stumptailed Macaques are hunted for food and traditional medicine (their bones), and live animals may enter local pet trades. They are classified variously as endangered or extremely threatened in China, with a population numbering ¢.3500 individuals. Overhunting, including exploitation for traditional food, has resulted in rapid shrinkage of its distribution in south-western China, and they may have been extirpated in Guangdong Province. Habitat loss from the exploitation of evergreen broadleaf forest is a serious threat. In the 1990s, the Stump-tailed Macaque was considered to be widespread above 11° N in central Vietnam and was classified as vulnerable. Earlier, it had been selected to be used in the production of polio vaccine. Poaching for traditional medicine, stimulated by increasing affluence in Vietnam and the East Asian region, is now putting extreme pressure on all primate populations in Vietnam, and no new conservation measures have been introduced. Direct threats to the habitat include logging, gold mining, and shifting cultivation by minority peoples. Elsewhere in the Indochinese Peninsula, development of the economy and infrastructure is resulting in habitat loss and degradation. In Cambodia, where the Stump-tailed Macaque is found in only three of 24 provinces, economic land and mining concessions occur even in protected areas. In Laos, Stump-tailed Macaques are common at only a few sites within its countrywide distribution. Large groups may be affected by opportunistic hunting to supply bones to Vietnam for traditional medicine. Terrestriality makes Stump-tailed Macaques more vulnerable to snaring than other species of macaques. Although still incipient, agriculture (particularly commodity crops), mining, and hydropower development are causing significant loss of forest habitat. The trend toward large-scale farms and plantations and use of marine products has resulted in serious habitat loss and degradation in peninsular Thailand. In 1964-1976, the USA—the world’s largest user of primates—imported ¢.30,000 primates from Thailand. Most were probably macaques, especially Stump-tailed Macaques, of which Thailand supplied more than 90% of the 2883 individuals imported by the USA in 1971 and 1972. Most were used in biomedical and other research, but some entered the pet trade as “miniature chimpanzees.” The population in peninsular Thailand was significantly reduced by trapping for this trade. In 1975, Thailand imposed a ban on the commercial export of macaques (and other primates), effective in April 1976, which virtually eliminated Stump-tailed Macaques from international trade.
Bibliography. Abegg & Thierry (2002a), Bauers (1993), Bertrand (1969), Chetry (2002), Chetry et al. (2002/2003), Chevalier-Skolnikoff (1975), Duckworth et al. (1999), Eudey (1979, 1980, 1981, 1987, 1991), Fooden (1967, 1990), Fooden et al. (1985), Groves (2001), Kingsada et al. (2010), Lekagul & McNeely (1988), Lim Boo Liat (1969), Molur et al. (2003), Mukherjee (1982), Thao et al. (2010), Trebouet et al. (2009), Vietnam, Ministry of Science, Technology and Environment (1992), Zhang Rongzu & Quan Guogiang (1996), Zhang Yongzu et al. (2002).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.