Macaca mulatta (Zimmermann, 1780)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6867065 |
|
DOI |
https://doi.org/10.5281/zenodo.6863178 |
|
persistent identifier |
https://treatment.plazi.org/id/CE199B17-FFD3-FFD7-FA2E-6F5DF73DF274 |
|
treatment provided by |
Jonas |
|
scientific name |
Macaca mulatta |
| status |
|
20. View Plate 36: Cercopithecidae
Rhesus Macaque
French: Macaque rhésus / German: Rhesusaffe / Spanish: Macaco Rhesus
Other common names: Rhesus Monkey
Taxonomy. Cercopithecus mulatta E. A. W. Zimmermann, 1780 ,
India, based solely on Tawny Monkey, a menagerie animal (presumably observed in London), not preserved, identified by Pennant in 1771.
Restricted by R. Pocock in 1932 to “Nepal Tarai” (= Terai), the narrow plain that extends along the southern border of Nepal. M. mulatta is a member of the fascicularis species group of macaques, including M. fascicularis , M. cyclopis , and M. fuscata , exhibiting the characteristic helmetshaped and relatively long and narrow glans penis. Assignment to a separate mulatta species group of macaques with M. cyclopis and M. fuscata also has been proposed. As many as ten subspecies have been recognized in classifications published since 1932: mulatta , sanctijohannis, lasiotus, tcheliensis, vestitus, villosus, littoralis, brevicaudus, siamica, and memahoni. Differentiation of local and regional populations, based on descriptions of traditional characters such as overall size, tail length, pelage color and length, and molecular diversity is regarded as inadequate by some recent authorities to warrant formal recognition of subspecies. The interspecific boundary with M. fascicularis extends ¢.2000 km across the Indochinese peninsula in the south-east. The two species share a considerable range between 17° N and 14° N in Vietnam and Laos (and probably Thailand). Hybridization has been inferred from morphological intermediates in absolute and relative tail length recorded in Thailand, Laos, and Vietnam and in some M. mulatta with noticeably less rufous hindquarters in southern Laos. Molecular studies differentiate three populations: Indian, Chinese, and Indochinese, the latter the apparent result of introgression from M. fascicular. Monotypic.
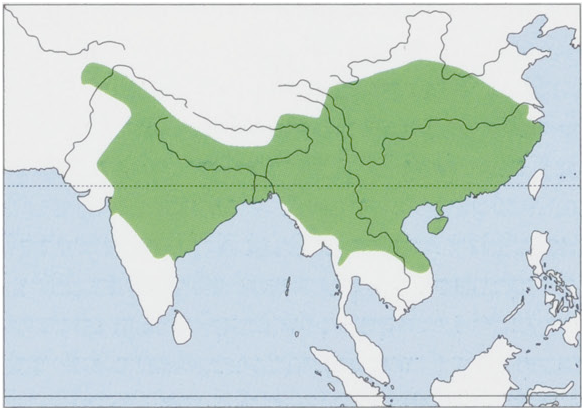
Distribution. S & SE Asia from ¢.36° N (in Afghanistan, Pakistan, India, and China) S to ¢.15° N (in India, Thailand, Laos, and Vietnam), also in Nepal, Bhutan, Bangladesh, Myanmar, and Hainan I. An isolated population at 40°24’ N in NE China was extirpated in 1987. View Figure
Descriptive notes. Head-body 41-66 cm (males) and 37-58 cm (females), tail 12-5— 31 cm (males) and 12.5-28 cm (females); weight 4-14.1 kg (males) and 3-10 kg (females). The Rhesus Macaque is an intermediate-sized member of the fascicularis species group. Head-body length and body weight tend to increase with latitude, while tail length generally decreases. High relative tail length (47-5-72-1%) in some southeastern specimens has been interpreted as evidence of hybridization with Long-tailed Macaques ( M. fascicularis ). Pelage of the upper back of the Rhesus Macaque varies from yellowish gray to golden brown to burnt orange with crown, nape, and sides of the head about the same color as the adjacent upper back. Pelage of the lower back varies from golden brown to burnt orange to intense burnt orange. A black streak marks the front edge of the crown, and blackish hairs may fringe cheeks. Crown hairs usually are smoothly directed backward. Hairs on sides of the head usually form a small crest or whorl near the angle of the jaw (infrazygomatic crest). Thinly haired facial skin is pale brown to reddish, except for upper eyelids that lack pigment. Dorsal pelage on proximal parts of limbs is similar in color to that on the adjacent trunk; pelage color of limbs is less erythristic distally. Base of the tail is approximately the same color as the lower back and becomes dark brown dorsally and buffy ventrally. Ventral surfaces of the trunk and limbs are thinly haired and pale buffy to whitish. The Rhesus Macaque undergoes seasonal molting and fading of pelage coloration. Pelage in Indian populations at upper elevations (2400 m) may be longer and sleeker than nearby populations at lower elevations. Abnormally pale “golden” individuals reportedly occur in various parts of India and Pakistan (and perhaps Thailand). Newborns have pale brown to dark brown pelage, generally darker than in adults, and a midline bare area in the crown hair. Bare skin of the face, hands, and feet changes from dull purple to pale pink within about five minutes of birth. Skin color changes to buffy by about two months of age, and the dark neonatal pelage is gradually replaced by paler pelage similar to that of adults, and without the midline part, by about six months of age.
Habitat. Deciduous and mixed deciduous/bamboo forest, scrub and secondary vegetation, and evergreen forest, in seasonal subtropical climates. The natural distribution of the Rhesus Macaque in the north extends to temperate and subalpine habitats, where forests are mixed broadleaf/needle-leaf and, less frequently, needle-leaf. It is highly adaptable and occurs in arid areas in western India and tidal swamps in eastern India and Bangladesh. Most elevation records are below 2000 m (below 600 m in Laos), but it occurs at ¢.3200 m in Nepal and ¢.4000 m in Qinghai Province, China. The Rhesus Macaque frequently inhabits disturbed areas, where it raids cultivated fields. In South Asia, especially India, it increasingly lives as a commensal with humans in urban and rural areas, and can be found at roadsides and temples.
Food and Feeding. Rhesus Macaques are primarily vegetarian, and their diets include fruits, seeds, flowers, leaves, buds, shoots, twigs, stems, roots, bark, pith, and gums from hundreds of species of angiosperms, gymnosperms, and fungi. Plant consumption varies geographically; as many as 150 wild plant species were recorded as food in northern India (Uttar Pradesh). Insects may be the most common animal food; other animal food includes spiders, crayfish, crabs, shellfish, fish, bird eggs, and honeycombs. Rhesus Macaques living in tidal swamp forest of the Sundarbans catch and eat fish on mudflats. Groups near human settlements opportunistically raid diverse cultivated crops and gardens and forage through garbage, while temple populations in India and Nepal may be provisioned.
Breeding. Reproductive behavior of the Rhesus Macaque is strongly seasonal in natural populations throughout its distribution, with mating peeking in autumn and winter and births occurring in spring and summer. Sexual maturity in females in natural populations usually does not occur until 3-5-5-5 years of age, although some may engage in fertile copulations as young as 2-5 years old. In captive females,first menstrual bleeding may occur about one year before sexual maturity. Males appear to become sexually mature somewhat later, usually at 6-5 years old. At ¢.5-5 years old, the testes permanently descend into the male’s scrotum. At or before this age, males generally leave their natal group. In postjuvenile females and males, regions of the skin undergo intermittent swelling and reddening associated with the reproductive cycle. In captive females, a pair of pinkish pubic swellings develops during the second or third year of life. Following the first menstrual bleeding, a large bilobed, blister-like pubic swelling extending backward to the sides of the vulva marks the beginning of puberty. During adolescence, which may last up to two years, the sexual skin becomes less acutely swollen but redder and more extensive, often extending to the root ofthe tail and over the buttocks and posterior surfaces of the thighs and iliac region. Sexually mature females experience reddening rather than swelling of the sexual skin, which frequently includes the face and nipples. Maximum intensity of the red color generally occurs near the day of ovulation and is retained during pregnancy and frequently lactation. Red sexual skin develops in about the same posterior and facial regions in pubertal and adolescent malesas in females, but there is no pubertal swelling. The red color becomes brighter in males during the mating season. Female sexual activity is cyclical in natural populations. The periovulatory period is 8-12 days, and ovulation appears to occur at the midpoint. Females average more than two receptive periods per mating season, and post-conception receptive periods occur also. Temporary mating associations or consortships are formed between receptive females and male partners. The duration of consortships varies from ¢.25 minutes to eleven days although 1-2 days may be typical, and females usually consort with more than one male. Consortships and mating may be initiated by either sex. A female typically presents her perineal region to the male who mounts her dorsoventrally. Usually, a series of mounts, separated by dismounts,is required to complete copulation. Coagulated semen or a “vaginal plug” may be visible on the female’s perineum following copulation. Male and female homosexual mounting and male masturbation have been observed in natural groups. The gestation period is 133-200 days and averages c.166 days. A single infant is born. Birth weight in one large laboratory averaged 476 g in 1067 female infants and 502-8 g in 1115 male infants; birth weight in natural populations may be less. Mean annual birth rate in natural populationsis 42-:9-90-8%. Infants may begin to take small amounts of solid food at two weeks of age. Mothers begin to resist nursing attempts about three months later, although nursing may extend through the subsequent mating and birth seasons and not be completed until the first infant is about one year old.. The greatest age at which a captive female is known to have produced a living infant is about 28 years and six months. Males may remain capable of copulatory ejaculation to c.30 years old. Maximum reported longevity in captivity is 37 years.
Activity patterns. Rhesus Macaques may spend as much as 72% of daylight hours on the ground and 28% in trees. Forest groups tend to be more arboreal than non-forest groups. Reportedly, they rarely descend from trees in the tidal swamp forest of the Sundarbans. In wild populations, average proportion of waking hours spent feeding is 15-8-45%, and feeding generally peaks in the morning after waking and in the afternoon before roosting for the night.
Movements, Home range and Social organization. Home range averaged c.65 ha in 323 non-forest groups of Rhesus Macaques in rural and urban areas and 196 ha in 129 forest groups. Overlap of home ranges of adjacent groups is extensive, and most contact between groups seems to provoke some degree of antagonism. Different parts of a group’s home range may be used during different seasons, depending on the availability of food and water. Daily movements averaged 1-2 km in nine non-forest groups and 1-9 km in an estimated 16 forest groups. The mean size of c.1182 non-provisioned or minimally provisioned groups was 32-2 individuals, with group sizes of two to ¢.250 individuals. Non-provisioned groups tend be the largest (86 to ¢.105 individuals) in the far north in Afghanistan and Qinghai and Henan provinces, China. Groups of Rhesus Macaques are multimale-multifemale, with resident lineages of female kin. Fission usually occurs between matrilines. Males usually leave their natal groups and join a nearby group at c.5 years 6 months old at or before permanent testis descent into the scrotum; subsequent transfers may occur from a few months to a few years later. The sex ratio of adult males to adult females is c.1:3. Solitary males have been observed throughout the distribution of the Rhesus Macaque.
Status and Conservation. CITES Appendix II. Classified as Least Concern on The IUCN Red List. Wild populations of Rhesus Macaques were threatened by trapping for export in South Asia during the mid-20" century. During 1956-1960, the USA annually imported an average of 120,000 Rhesus Macaques from India, following a 1955 agreement between the two countries permitting their export for use in development and testing of polio vaccine and for other medical uses. An annual export quota of 50,000 individuals was subsequently established. In the state of Uttar Pradesh, the only area for which data on population trends are available, intensive trapping for export caused a conspicuous change in the age structure of the populations of Rhesus Macaques , with juveniles experiencing the greatest decline. Further reductions in the export quota were followed by an Indian ban on the export of all primates, effective in 1978, as the Indian governmentcited the USA for using Rhesus Macaques in militaryrelated research. During 1964-1978, the USA—the world’s largest user of primates— imported a total of 332,000 primates from India, of which more than 99% probably were Rhesus Macaques. China began captive breeding of native Rhesus Macaques in 1978 and was exporting them by 1984. Molecular differences between Chinese and Indian macaques resulted in renewed interest by Western researchers in obtaining the latter. Efforts to establish breeding colonies in Nepal were abandoned because of local protest. Hunting, habitat loss, forestfires, accidental mortality, and changes in native species dynamics formerly posed threats. Subsistence hunting still occurs in northeastern and central India and mid-central Nepal, and there is some trade for pets and entertainment. Some trapping for a pet trade may also occur in eastern Afghanistan.
Currently, the main threat is deterioration and loss of habitat and increasing conflict with people, especially in South Asia. The Indian government has invoked a policy of “zero tolerance” toward pest behavior in urban areas. In 2007, the northern Indian state of Himachal Pradesh embarked on a program of conflict management that includes masssterilization of Rhesus Macaques.
Bibliography. Abegg & Thierry (2002a), Brandon-Jones et al. (2004), Chaudhuri & Sinha (2010), Duckworth et al. (1999), Eudey (1979, 1980), Fooden (1971, 2000, 2006), Fooden et al. (1981), Groves (2001), Hamada, Kawamoto, Kurita et al. (2009), Hamada, Kawamoto, Oi et al. (2009), Hasan et al. (2013), Jadejaroen et al. (2010), Jintanugool et al. (1985), Kingsada et al. (2010), Lekagul & McNeely (1988), Lindburg (1971, 1977), Mack & Eudey (1984), Majumder et al. (2012), Molur et al. (2003), Mukherjee (1982), Rattan (2010), Southwick & Lindburg (1986), Southwick & Siddigi (1977), Southwick et al. (1983), Stevens et al. (2011), Teas et al. (1980), Thao et al. (2010), Vietnam, Ministry of Science, Technology and Environment (1992), de Vries & Shah (2010), Zhang Rongzu & Quan Guogiang (1996), Zhang Yongzu et al. (2002).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.