Moina australiensis Sars, 1896
|
publication ID |
https://doi.org/10.11646/zootaxa.4577.1.10 |
|
publication LSID |
lsid:zoobank.org:pub:101586AC-25B7-4D12-AB05-80A0A504C528 |
|
DOI |
https://doi.org/10.5281/zenodo.5931102 |
|
persistent identifier |
https://treatment.plazi.org/id/D00CB577-9931-FA3D-FF55-3566B873FCF2 |
|
treatment provided by |
Plazi |
|
scientific name |
Moina australiensis Sars, 1896 |
| status |
|
Moina australiensis Sars, 1896 View in CoL
( Figs. 1–10 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 , 11A, B View FIGURE 11 )
Moina australiensis Sars 1896: p. 18 View in CoL –24, pl. 3, fig. 1–11.
Moina australiensis in Brehm 1953: p. 6 View in CoL –9, fig. 2a–e.
Moina australiensis in Goulden 1968: p. 48 View in CoL –51, fig. 21a–j.
Etymology. Sars named this species in honor of Australia, the continent where it was found ( Sars 1896).
Type locality. Sydney , New South Wales, Australia ( Sars 1896) .
Type material. Potential author’s type material is kept at Zoological Museum, Oslo, Norway (GOS F 19249 and 19250) .
Material examined from terra typica, Australia. GoogleMaps Over 100 parthenogenetic females, 50 ephippial females and 20 males from a pond (S36.365645°, E148.934131°) near Carrolls Lake, limnetic, 10 km E Berridale, New South Wales, Australia, coll. 13.05.1975 by B.V. Timms, NNS-1997-217; over 100 parthenogenetic females, 50 ephippial females and 30 males from Boundary Lake GoogleMaps (S36.538924°, E149.119192°), 18 km W Nimmitabel, near Cooma GoogleMaps , Monaro Table GoogleMaps land, New South Wales, Australia, coll. 14.05.1975 by B.V. Timms, NNS-1997-223.
Short diagnosis. Species of large size for the genus (length of adult parthenogenetic female up to 1.30 mm). Parthenogenetic female with body typical for the genus. Head and valves covered by fine hairs. Head without rostrum and head pore, ocellus absent. On inner side of valve, setulae near ventral setae are grouped into prominent bunches. Preanal and postanal margins of postabdomen covered by fine, relatively long hairs. Lateral side of postabdomen with a row of denticles decreasing in size towards tip. Antenna I relatively thick, its length almost five diameters of base. Structure of antenna II, maxilla I and thoracic limbs as for the genus. Ephippium darkbrownish, containing two resting eggs. Its dorsal chitinous plate wrinkled. Macrosculpture of ephippium represented by protruding knobs, some of them rugged. Male with elongated body. Gonopore opening in ventral surface of postabdomen near base of claws. Antenna I long, with four bisegmented hooks of similar size. Thoracic limb I with a long exopodite. Stiff seta 1 of male limb I having more thick basal segment than in female.
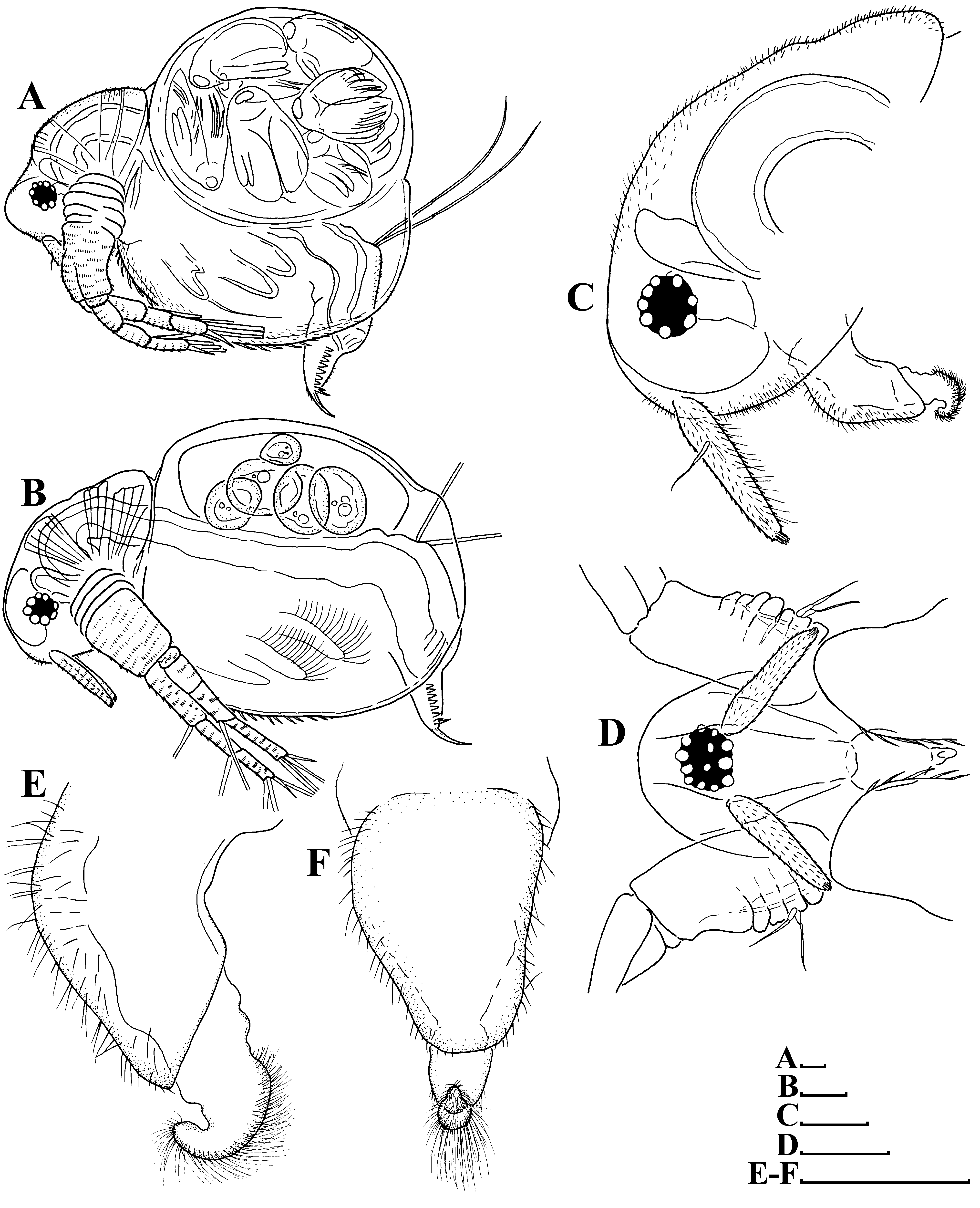
Redescription. Parthenogenetic female ( Figs. 1 View FIGURE 1 A–F, 2A–K, 3A–I, 4A–G, 5A–I, 11A–B). General. In lateral view, body broadly ovoid ( Fig. 1A View FIGURE 1 ) or subovoid ( Fig. 1B View FIGURE 1 ), maximum height in body middle (body height/length ratio about 0.85 in adults). Body shape may vary significantly due to increasing of brood pouch volume during development of embryos ( Figs. 1 View FIGURE 1 A–B). Dorsal margin convex, with a deep depression between head and valves. Posterodorsal angle broadly rounded, posteroventral angle well visible, triangular. Anteroventral angle broadly rounded ( Figs. 1 View FIGURE 1 A–B). Fine short hairs on head and valves ( Figs. 1A, C View FIGURE 1 , 4B View FIGURE 4 ).
Head ( Figs. 1 View FIGURE 1 A–D, 4A, 11A) relatively large, broadly ovoid in lateral view ( Figs. 1 View FIGURE 1 A–C, 4A), without a rostrum, and with a distinct supraocular depression ( Fig. 1A View FIGURE 1 , 11A View FIGURE 11 ). No ocellus near base of antenna I ( Figs. 1 View FIGURE 1 A–C, 11A). A shallow dorsolateral depression in posterior portion of head. Lateral, dorsal and frontal head pore absent. Dorsal and lateral surfaces of head covered by fine short hairs ( Figs. 1A, C View FIGURE 1 , 11A View FIGURE 11 ).
Labrum ( Figs. 1 View FIGURE 1 E–F) with fleshy main body, its ventral margin convex, labral plate densely setulated.
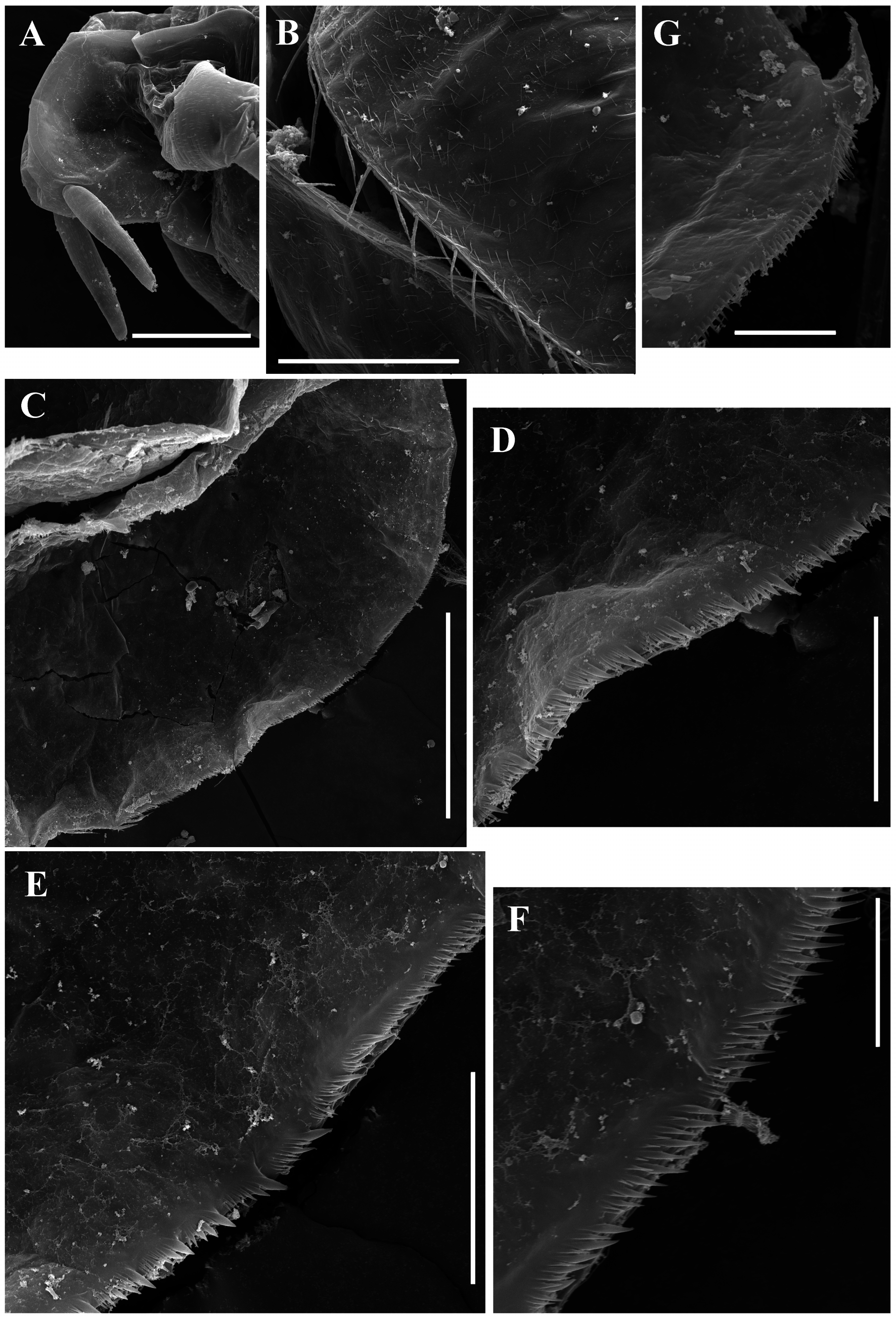
Valves ( Figs. 1 View FIGURE 1 A–B) large, broadly ovoid. Ventral margin of valve broadly rounded, convex, with short setae, decreasing in size posteriorly ( Figs. 2 View FIGURE 2 A–B, 3A–B). Posterior portion of ventral margin with a row of fine, exactly marginal setules ( Figs. 3 View FIGURE 3 B–D, 4C–F). Armature of this portion is significantly different in the different regions ( Fig. 4C View FIGURE 4 ). Bunches of setulae adjoin to ventral setae ( Figs. 2 View FIGURE 2 B–C, 3B–C, 4D–E, 11B); posterior setule in each bunch especially thick and long ( Figs. 4 View FIGURE 4 D–E). Setulae located closer to the dorsal margin subequal in thickness, decreasing in length posteriorly ( Figs. 2D View FIGURE 2 , 3D View FIGURE 3 , 4F View FIGURE 4 ). A setulated spine on inner face of valve near posteroventral angle ( Figs. 2E View FIGURE 2 , 3E View FIGURE 3 , 4G View FIGURE 4 ). On outer surface valves show prominent sculpture represented by elongated polygons ( Figs. 4B View FIGURE 4 ) and fine short hairs.
Thorax ( Figs. 1 View FIGURE 1 A–B) long; abdomen relatively short.
Postabdomen ( Fig. 2F View FIGURE 2 ) triangular, elongated, conically narrowing distally. Postabdomen length/height ratio about 2.8. Ventral margin almost straight, covered by rows of fine setulae ( Figs. 2 View FIGURE 2 F–G, 3F). Anus located almost in middle of postabdomen. Preanal margin long, broadly convex ( Fig. 2F View FIGURE 2 ), covered by fine relatively long hairs. Anal margin almost straight, almost two times shorter than postanal margin. Preanal and postanal angles smooth. Postanal margin covered by bunches of fine hairs ( Figs. 2 View FIGURE 2 F–G, 3F). Basis of claws not inflated ( Figs. 2 View FIGURE 2 F–G, 3F–G). In lateral face, postanal margin bears a large bident tooth (its branches always unequal in length) and a row of 11– 13 large, triangular plumose teeth ( Figs. 2 View FIGURE 2 F–G, 3F).
Postabdominal seta ( Fig. 2F View FIGURE 2 ) long, subequal in length to postabdomen; its distal segment covered by fine hairs.
Postabdominal claw ( Figs. 2G View FIGURE 2 , 3F View FIGURE 3 ) robust, long (almost subequal in length than postanal margin), slightly curved, with particularly sharp tip. Its outer lateral side with a row of denticles decreasing in size towards tip. Prominent group of larger denticles near base of claw. Ventral margin of claw with about several denticles in its basal portion.
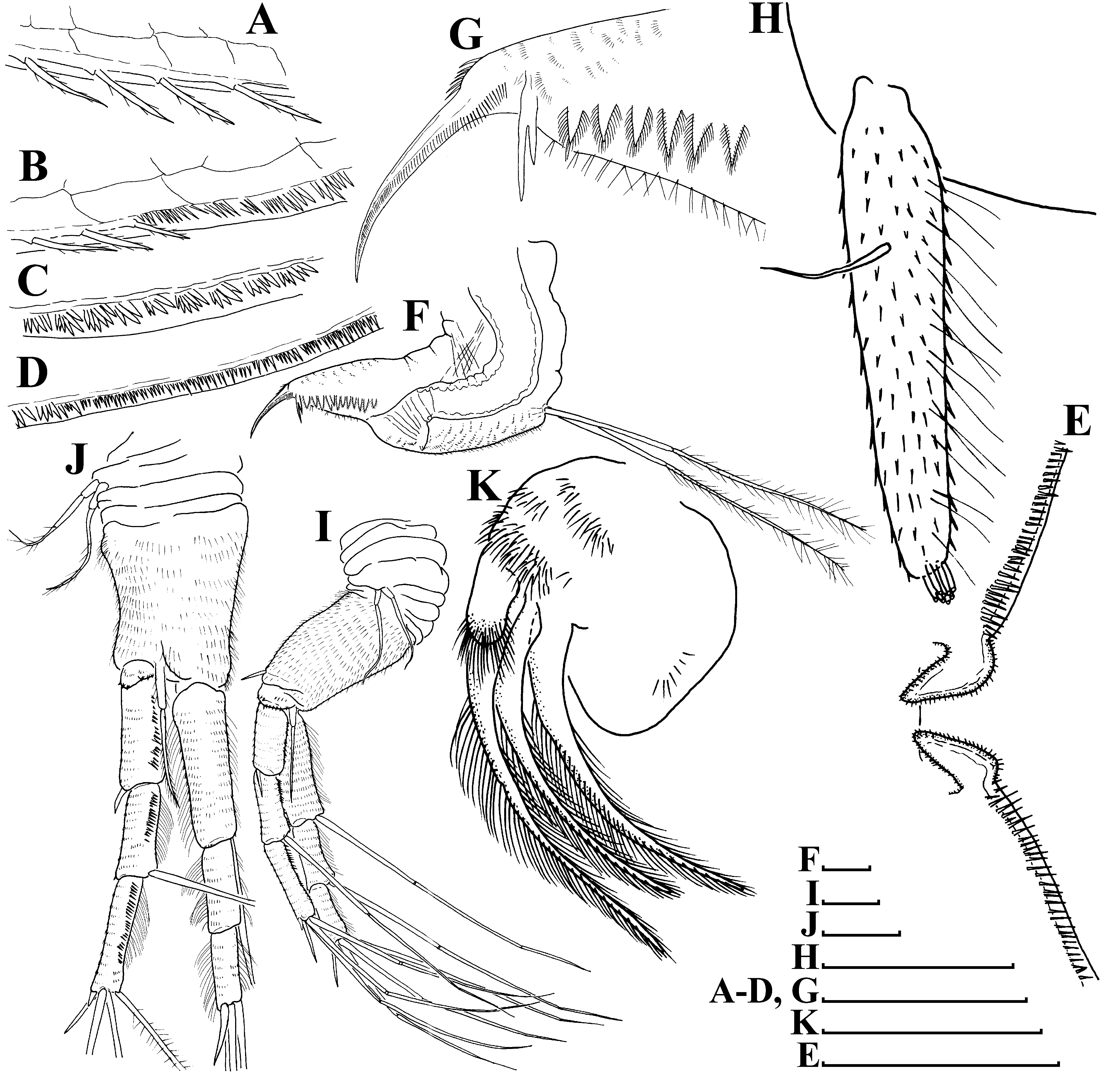
Antenna I ( Figs. 2H View FIGURE 2 ) almost cylindrical, covered by concentric rows of minute denticles and fine long hairs. Antennular sensory seta slender, arising somewhat proximally to middle of antennular body on its lateral face. Nine short aesthetascs subequal in length.
Antenna II ( Figs. 2 View FIGURE 2 I–J) long. Coxal part with two setulated sensory setae subequal in length. Basal segment robust, with a short distal spine at outer face and a long seta at inner face ( Figs. 2 View FIGURE 2 I–J). Antennal branches elongated, four-segmented exopod slightly longer than three-segmented endopod, all their segments cylindrical, with concentric rows of fine denticles and long fine hairs. Antennal formula: setae 0-0-1-3/1-1-3, spines 0-1-0-1/0-0-1. Apical and lateral setae of endopod and exopod have similar armature: all with basal and distal segments bilaterally covered by fine setulae ( Fig. 2J View FIGURE 2 ). Spine on second exopod segment has same length as spine between exopod and endopod branches ( Fig. 2I View FIGURE 2 ). Apical spines of exopod and endopod subequal in length ( Figs. 2 View FIGURE 2 I–J).
Maxilla I ( Fig. 2K View FIGURE 2 ) with three long densely setulated setae.
Thoracic limbs ( Figs. 3 View FIGURE 3 H–I, 5A–I): five pairs.
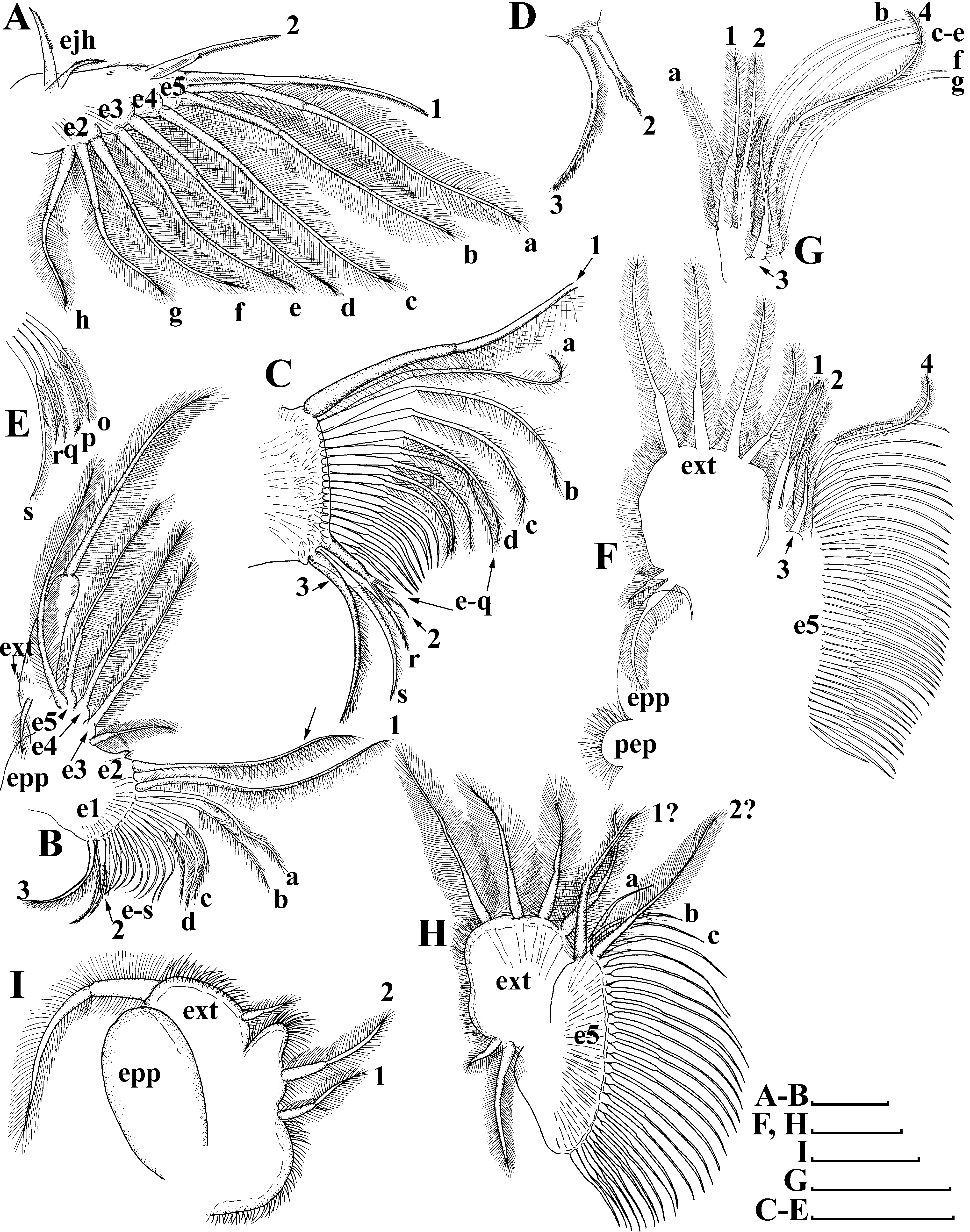
Limb I ( Figs. 3 View FIGURE 3 H–I, 5A) with elongated corm, inner distal lobe, or endite 5 (sensu Kotov 2013), with a single anterior seta ( Fig. 5A View FIGURE 5 : 1), bearing short setulae, and two soft setae ( Fig. 5A View FIGURE 5 : a, b). Armature of posterior soft setae similar in all limbs: both their segments armed with fine, long setulae ( Fig. 5A View FIGURE 5 : a–h). Endite 4 with a single, relatively short anterior seta ( Fig. 5A View FIGURE 5 : 2), and a single posterior seta ( Fig. 5A View FIGURE 5 : c). Endite 3 with two posterior setae ( Fig. 5A View FIGURE 5 : d–e), without anterior setae. Endite 2 with three posterior setae ( Fig. 5A View FIGURE 5 : f–h). Two ejector hooks of remarkably different size. No maxillar process (endite 1) on limb base.
Limb II ( Figs. 5 View FIGURE 5 B–E) with ovoid epipodite, limb distal portion as a large lobe bearing a large soft seta and a small lateral seta ( Fig. 5B View FIGURE 5 ); three endites bearing four soft posterior setae ( Figs 5B View FIGURE 5 : e5–e3), and two small setae of unclear homology near gnathobase. Gnathobase with two clear rows of setae: four anterior setae; a single long seta ( Fig. 5C View FIGURE 5 : 1) near the beating seta (marked via arrows without number on Figs. 5 View FIGURE 5 B–C) and two setae in the basal corner of the gnathobase ( Figs. 5C, D View FIGURE 5 : 2–3), increasing in size distally; and nineteen posterior setae ( Figs. 5 View FIGURE 5 B–C, E: a–s) of the gnathobase “filter plate”.
Limb III ( Figs. 5 View FIGURE 5 F–G) with setulated preepipodite and ovoid epipodite. Exopodite large, ovoid, bearing four distal and two lateral setae ( Fig. 5F View FIGURE 5 ). Inner distal portion of limb with three endites: endite 3 with a single anterior ( Fig. 5G View FIGURE 5 : a) and a single posterior seta ( Fig. 5G View FIGURE 5 : 1); endite 2 with single anterior ( Fig. 5G View FIGURE 5 : 2) and two posterior setae ( Fig. 5G View FIGURE 5 : b–c); endite 1 with a single short anterior ( Fig. 5G View FIGURE 5 : 3) and four posterior setae ( Fig. 5G View FIGURE 5 : d–g). Long anterior seta ( Fig. 5G View FIGURE 5 : 4) located in the distal corner. All other parts of limb inner margin represent a single large lobe (probably this is a gnathobase (Kotov 2013)), bearing numerous setae ( Fig. 5G View FIGURE 5 : endite 5).
Limb IV ( Fig. 5H View FIGURE 5 ) smaller than limb III. Exopodite, similar to that in limb III, also bearing four distal and two lateral setae. Inner distal portion of this limb with two endites: endite 2 with an anterior seta ( Fig. 5H View FIGURE 5 : 1) and a posterior seta ( Fig. 5H View FIGURE 5 : a); and endite 1 with an anterior seta ( Fig. 5H View FIGURE 5 : 2) and two posterior setae ( Fig. 5H View FIGURE 5 : b–c). The major portion of the limb inner margin is the gnathobase filter plate (Kotov 2013), consisting of numerous setae ( Fig. 5H View FIGURE 5 : endite 5).
Limb V ( Fig. 5I View FIGURE 5 ) with large ovoid epipodite. Exopodite broadly ovoid, provided with a long distal and a small apical seta. Inner limb portion as a flat, setulated subrectangular lobe; and two setae unequal in size ( Fig. 5I View FIGURE 5 : 1, 2).
Ephippial female ( Figs. 6A View FIGURE 6 , 7A View FIGURE 7 , 8 View FIGURE 8 A–H, 9A–E). General body shape and appendages of ephippial female appearing similar to those of parthenogenetic female ( Figs. 8 View FIGURE 8 A–D), but with dorsal portion modified into ephippium, containing two resting eggs (e.g. Fig. 6A View FIGURE 6 ). Dorsal part of valves with reinforced dorsal wrinkled chitinous plate ( Figs. 7A View FIGURE 7 , 8E View FIGURE 8 , 9 View FIGURE 9 A–E). In dorsal view, this plate has the same thickness almost all over the ephippium ( Fig. 9B View FIGURE 9 ). Ephippium (after formaldehyde fixation, living animals were not investigated by us) darkbrownish ( Fig. 7A View FIGURE 7 ). Macrosculpture of ephippium represented by protruding knobs, well-recognizable both under light ( Figs. 6A View FIGURE 6 , 7A View FIGURE 7 ) and scanning electron (8E–H, 9A–E) microscopes; such knobs near anterior, ventral and posterior portions of ephippium significantly lower than in the central portion ( Figs 8E, H View FIGURE 8 , 9 View FIGURE 9 A–C). Microsculpture as tiny irregular wrinkles on knobs, visible only under scanning electron microscope ( Figs. 8 View FIGURE 8 F–H, 9D–E).
Male ( Figs. 6 View FIGURE 6 B–K, 7B–D, 10A–C). General. In lateral view body ovoid, more elongated as compared to female (body height/length about 0.56) ( Fig. 6B View FIGURE 6 ). Dorsal margin of valves slightly elevated above head, posteroventral angle distinct ( Fig. 6B View FIGURE 6 ).
Head ( Figs. 6 View FIGURE 6 B–D) more elongated than in female. Labrum similar to females, with large, setulated distal labral plate ( Figs. 6 View FIGURE 6 E–F). Head pores absent. Compound eye large. Ocellus absent.
Valves ( Fig. 6B View FIGURE 6 ) ovoid, longer than that in female, with short setae, decreasing in size posteriorly, along ventral margin ( Figs. 6G View FIGURE 6 ). Posterior portion of ventral margin with successive series of fine, exactly marginal setulae ( Figs. 6G View FIGURE 6 ).
Thorax ( Fig. 6B View FIGURE 6 ) relatively long.
Abdomen ( Fig. 6B View FIGURE 6 ) short.
Postabdomen ( Figs. 6 View FIGURE 6 H–I) generally as in female, with a large bident tooth distally (distal branch always significantly large than proximal branch), and row of about 9–11 large, triangular plumose teeth ( Figs. 6 View FIGURE 6 H–J). Gonopores open in ventral surface of postabdomen near base of claws ( Figs. 6 View FIGURE 6 H–J).
Postabdominal setae ( Figs. 6B, H View FIGURE 6 ) almost two times longer than postabdomen. Its distal segment significantly longer than proximal one ( Fig. 6H View FIGURE 6 ).
Antenna I ( Figs. 6 View FIGURE 6 C–D) very long and regularly curved, covered by tiny hairs and rows of small denticles ( Figs. 6 View FIGURE 6 C–D). Antennular sensory seta long, arising from first quarter of antennular body. Male seta more robust, located near sensory seta. Apical tip of antennular body separated into two parts: first one with nine short aesthetascs ( Figs. 6 View FIGURE 6 C–D, K, 7B–C, D), second one with four bicuspid hooks ( Figs. 6 View FIGURE 6 C–D, K, 7B–C, D).
Limb I ( Figs. 10 View FIGURE 10 A–C) essentially similar to parthenogenetic female, but with large, curved copulatory hook ( Figs. 10 View FIGURE 10 A–B) and long exopodite ( Figs. 10 View FIGURE 10 A–C). Exopodite bisegmented, with proximal portion covered by fine relatively long hairs and distal portions with small fine denticles ( Fig. 10C View FIGURE 10 ). Stiff seta 1 ( Figs. 10 View FIGURE 10 A–B) has the basal segment thicker than in females.
Size. Adult parthenogenetic females up to 1.30 mm in length; ephippial females up to 1.10 mm in length; adult males up to 0.80 mm in length.
Variability. No significant variability between investigated populations was found.
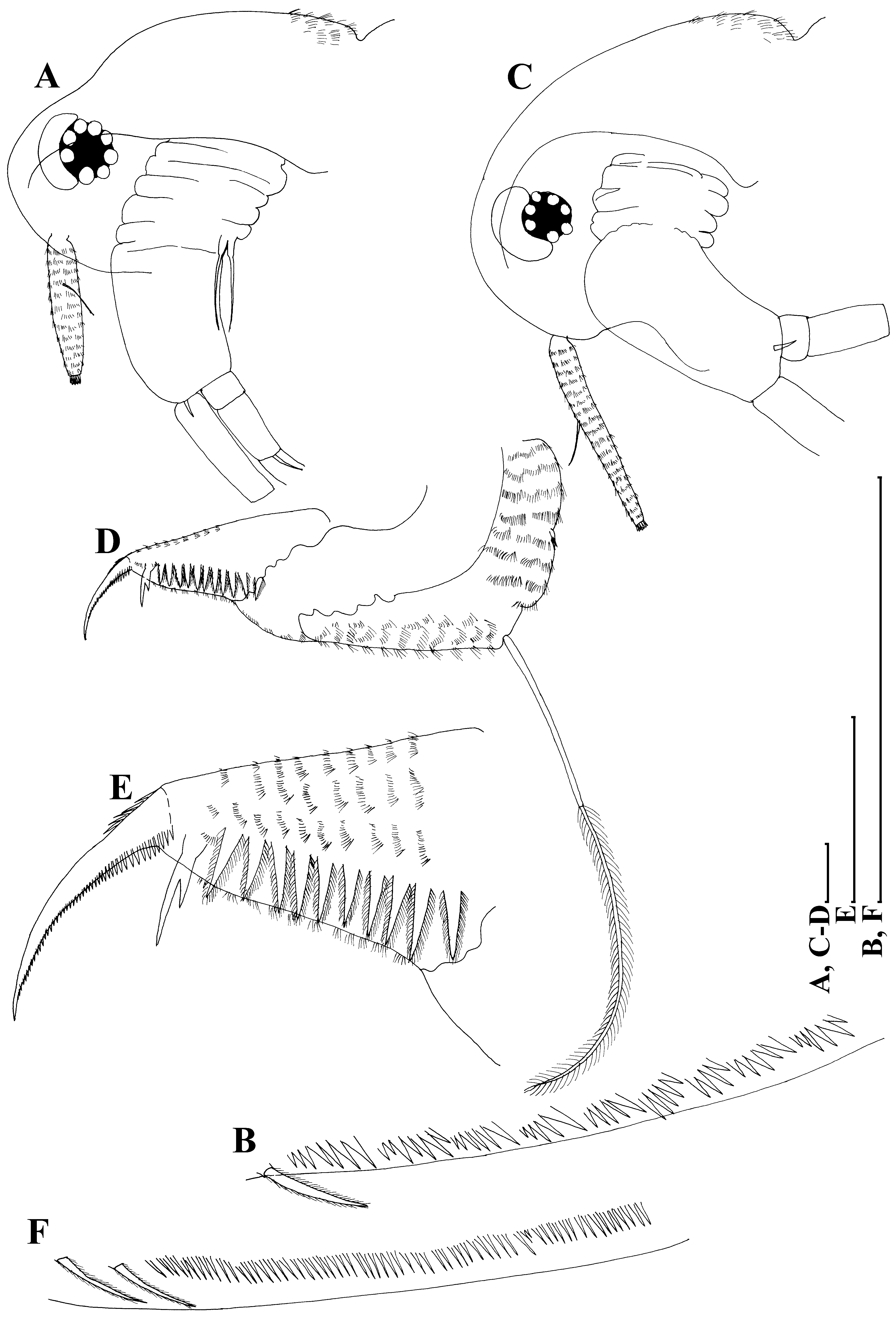
Taxonomic remarks. Among Australian moinids, only Moina tenuicornis Sars, 1896 may be considered as a congener of M. australiensis ( Figs. 11 View FIGURE 11 A–F). Both species share some fine, but very consistent morphological features (see detailed comparison in Goulden (1968) and Smirnov (1976)), among them: (1) lack of a dorsal head pore ( Figs. 11A, C View FIGURE 11 ); (2) distal portion of postanal margin with bunches of fine hairs ( Figs. 2F, G View FIGURE 2 , 3F View FIGURE 3 , 8B View FIGURE 8 , 11 View FIGURE 11 D–E); (3) ephippium with two resting eggs. But they reliably differ from each other in: (1) proportions of antenna I and (2) structure of inner margin of valves. M. australiensis has a relatively short antenna I, with a length reaching almost five diameters of the base ( Figs. 11A View FIGURE 11 ), while M. tenuicornis has elongated antenna I, with a length reaching eight diameters of base ( Figs. 11C View FIGURE 11 ). In M. australiensis setulae near ventral setae are grouped into bunches ( Fig. 11B View FIGURE 11 ), while in M. tenuicornis these setulae form a uniform row ( Fig. 11F View FIGURE 11 ).
Ecology and distribution. M. australiensis was found to date in brackish water lakes with low turbidity ( Timms 1993) and flooded rock holes with a broad range of physicochemical characteristics ( Bayly et al. 2011). At the same time, M. tenuicornis was found in sewage ponds (Wellington, New Zealand), characterized by slightly alkaline water, dissolved oxygen concentration more than of 6 mg /liter and high concentrations of organic substances ( Vidal 1973). Relatively similar conditions plus especially high turbidity were reported by Shiel et al. (1982). Probably, these two species differ in their ecological requirements, but further investigations are required on their preferences. Except for Vidal (1973), other publications with detailed descriptions of ecological conditions do not contain figures or microphotographs of investigated populations ( Shiel et al. 1982; Timms 1993, 1997; Bayly et al. 2011) and we could not check accurateness of author’s identification.
According to Goulden (1968) and Smirnov (1976), distribution ranges of M. australiensis and M. tenuicornis are mainly restricted to Australia. There are no reliable data on occurrence of these taxa in other parts of Australasia or in the Oriental zone. Sars’ record of M. tenuicornis in South Africa ( Sars 1916: pl. XXXV 2, 2a, 2b, 2c) seems doubtful in the frame of the continental endemism concept ( Frey 1982), although a specimen illustrated by him has a relatively long antenna I ( Sars 1916: pl. XXXV 2, 2a). Some other authors have recorded M. tenuicornis in Africa (e.g. Smirnov 1976; Clarke & Rayner 1999; Seaman et al. 1999). They pictured specimens with a relatively long antenna I as well (e.g. Seaman et al. 1999: fig. 4.8.: p), but, unfortunately, their descriptions lack data on the head pores, armature of valve, structure of distal portion of postanal margin and ephippium ornamentation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Moina australiensis Sars, 1896
| Neretina, Anna N. & Kirdyasheva, Anna G. 2019 |
Moina australiensis
| in Goulden 1968: 48 |
Moina australiensis
| in Brehm 1953: 6 |
Moina australiensis
| Sars 1896: 18 |