Glossocephalus milneedwardsi Bovallius, 1887
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4027.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:B6DDF93A-BED1-495E-BFFD-ABB51310A1E8 |
|
DOI |
https://doi.org/10.5281/zenodo.6100457 |
|
persistent identifier |
https://treatment.plazi.org/id/D067DC71-FFB2-B611-FF1F-FF5EDCFD5149 |
|
treatment provided by |
Plazi |
|
scientific name |
Glossocephalus milneedwardsi Bovallius, 1887 |
| status |
|
Glossocephalus milneedwardsi Bovallius, 1887 View in CoL
( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Glossocephalus Milne-Edwardsi Bovallius, 1887: 35 View in CoL .— Bovallius 1890: 105 (key), 106–108, pl. 5, fig. 5; text figs. 6, 6a (p. 22), 71 (p. 38). Chevreux 1913: 11 –15, figs. 4–5. Chevreux & Fage 1925: 433, fig. 421. Chevreux 1935: 200 –202, pl. 14, figs. 1–2.
Glossocephalus Milne-Edwardsii View in CoL — Colosi 1918: 221. Stephensen 1925: 202 –203, 230 (table). Cecchini 1929: 485, fig. 4.
Glossocephalus Milne-Edwarsi View in CoL (typographical error)— Pirlot 1938: 43. Pirlot 1939a: 55. Pirlot 1939b: 78 –79.
Glossocephalus milne -edwardsi — Spandl 1927: 196 (key), 196–197, fig. 24. Barnard 1931: 131 –132. Barnard 1937: 193. Barnard 1940: 485. Shoemaker 1945: 253, fig. 45. Irie 1959: table 4, 31 (table). Fage 1960: 83 –87, text figs. 56, 57 (map), 140–141 (distribution table). Pillai 1966: 186 –187, fig. 10; pl. 1, fig. J. Dick 1970: 42 (key), 70, fig. 14 (part). Yoo 1971: 43 (list), 68. Bowman & Gruner 1973: 51, fig. 68. Zeidler 1978: 34, fig. 33. Laval 1980: 20, 21 & 23 (tables). Lin & Chen 1994: 114, 119 (table). Lin et al. 1995: 123 (table). Shih & Chen 1995: 200 –202, fig. 132. Lin et al. 1996: 231 (table). Zelickman 2005: xvii (list), figs. 53a, b (pp. 324–327).
Glossocephalus milneedwardsi View in CoL — Tashiro & Jossi 1972: 12 (map), 21 (list). Thurston 1976: 438. Vinogradov et al. 1982: 427 – 428, fig. 229. Zeidler 1984: 298, fig. 9d (abundance), 301. Nair & Jayalakshmy 1992: passim. Nair 1995: 19, pl. 3b, figs. N1 & N2; pl. 14. Lowry 2000: 327 (table). Gasca & Shih 2001: 496 (table). Escobar-Briones et al. 2002: 367 (table). Gates et al. 2003: 328. Brusca & Hendrickx 2005: 152 (list). Browne et al. 2007: 819 (list), fig. 4 (phylogenetic tree). Garcia- Madrigal 2007: 156 –157, 192 (table). Gasca 2007: 118 (table). Gasca & Franco-Gordo 2008: 569 (table). Gasca 2009a: 89 (table). Gasca 2009b: 66 (table). Gasca 2009c: 218 (table). Gasca et al. 2009: 1497 (table). Lavaniegos & Hereu 2009: 142 (table), 152 (appendix). Valencia & Giraldo 2009: 268 (table). Mori et al. 2010: 10 (list). Gasca et al. 2012: 126 (table). Valencia & Giraldo 2012: 1493 (table). Hurt et al. 2013: 31 (table), figs. 1–2 (phylogenetic trees).
Glossocephalus milne -edwardsii — Hure et al. 1969: 603 & 605 (tables). Harbison et al. 1977: 479 (table), 480, 483 (table). Harbison et al. 1978: 239, 251 (table).
Glossocephalus milneedwardsii — Brusca 1981: 12 (list), 45, figs. 22a, b.
Glossocephalus spiniger Bovallius 1887: 35 .— Bovallius 1890: 105 (key), 108–110, pl. 5, figs. 6–9; text figs. 26 (p. 26), 43 (p. 29). Steuer 1911a: 352 (key), fig. 1. Steuer 1911b: 683 (key). Spandl 1927: 196 (key).
Glossocephalus adriaticus Steuer, 1911a: 352 (key), fig. 2 (“adriatischen form”).— Steuer 1911b: 682 –685, pl. 3, figs. 1–7.
Elsia indica Giles, 1890: 250 View in CoL –251, pl. 6, figs. 2–4.— Walker 1904: 237 –238, pl. 1, fig. 2.
Type material. Type material of G. milneedwardsi Bovallius, 1887 , consisting of the remains of two male specimens ( Fig. 1 View FIGURE 1 ), is in the NRS (reg. no. 8706 & 8707), as detailed above. It is most likely that the former specimen is the one illustrated by Westergren ( Bovallius 1890), and it is here designated the lectotype. The other material, consisting only of male second antennae, becomes paralectotype material. Bovallius (1887, 1890) only provides a general locality, “tropical region of the Atlantic” and mentions that he had both male and female specimens.
Type material of synonyms. Type material of G. spiniger Bovallius, 1887 , consisting of a partly dissected female ( Fig. 2 View FIGURE 2 ), is in the ZMUC (CRU-9424), labelled “Indian 7–2°S 80–90°E CASPERSEN 1869”, which is consistent with the type locality provided by Bovallius (1887, 1890). It seems likely that this is the specimen illustrated by Bovallius (1890), and is here regarded the holotype because the data provided by Bovallius implies that he only had one specimen, a female about 11 mm.
The unique type female (3.3 mm) of G. adriaticus Steuer, 1911 could not be found in any major European institution and is considered lost. The type locality is the north-east Adriatic Sea, near “Rovigno” (= Rovinj), on the ctenophore Deiopea kaloktenota , collected by T. Krumbach, December 1910. The type is a juvenile specimen, and despite the inadequate description, the figures provided by Steuer (1911a, 1911b) are clearly representative of typical specimens of G. milneedwardsi .
The unique type female (4.0 mm) of Elsia indica Giles, 1890 could not be found in the NHM, London and is considered lost. The type locality is Bombay Harbour, surface, Investigator expeditions, A. Carpenter commanding. The type is a juvenile specimen and is a most likely synonym of G. milneedwardsi , considering the description and figures provided by Giles (1890).
Material examined. The remains of the lectotype and paralectotype of G. milneedwardsi and the holotype of G. spiniger as detailed above. In addition the following specimens.
N.W. Atlantic: One female, one male ( SAMA C5815), South of Georges Bank [38°N 67°W], G. Matsumoto, scuba, 28 July 1987. N.E. Pacific: Two females, one male ( SAMA C5816), off California [33°27’N 110°23’W], G. Matsumoto, scuba, 1985. One male ( SAMA), south of the Gulf of Alaska [50°12.23’N 144°48.11’W], 1000–0 m, Line P Monitoring Program, 11 June 2005. S.W. Pacific: Ten females, five males ( SAMA), Great Barrier Reef region, off Townsville, Queensland ( Zeidler 1978). N.E. Indian: One female ( SAMA), off Exmouth, Western Australia [21°49.86’S 114°30.31’E], 10 February 1999. S.W. Indian: Ten females, eight males, seven juveniles ( SAM), off South Africa, between Durban and East London, and Durban and Kosi Bay, Meiring Naude cruises, 1976, 1977, 1979.
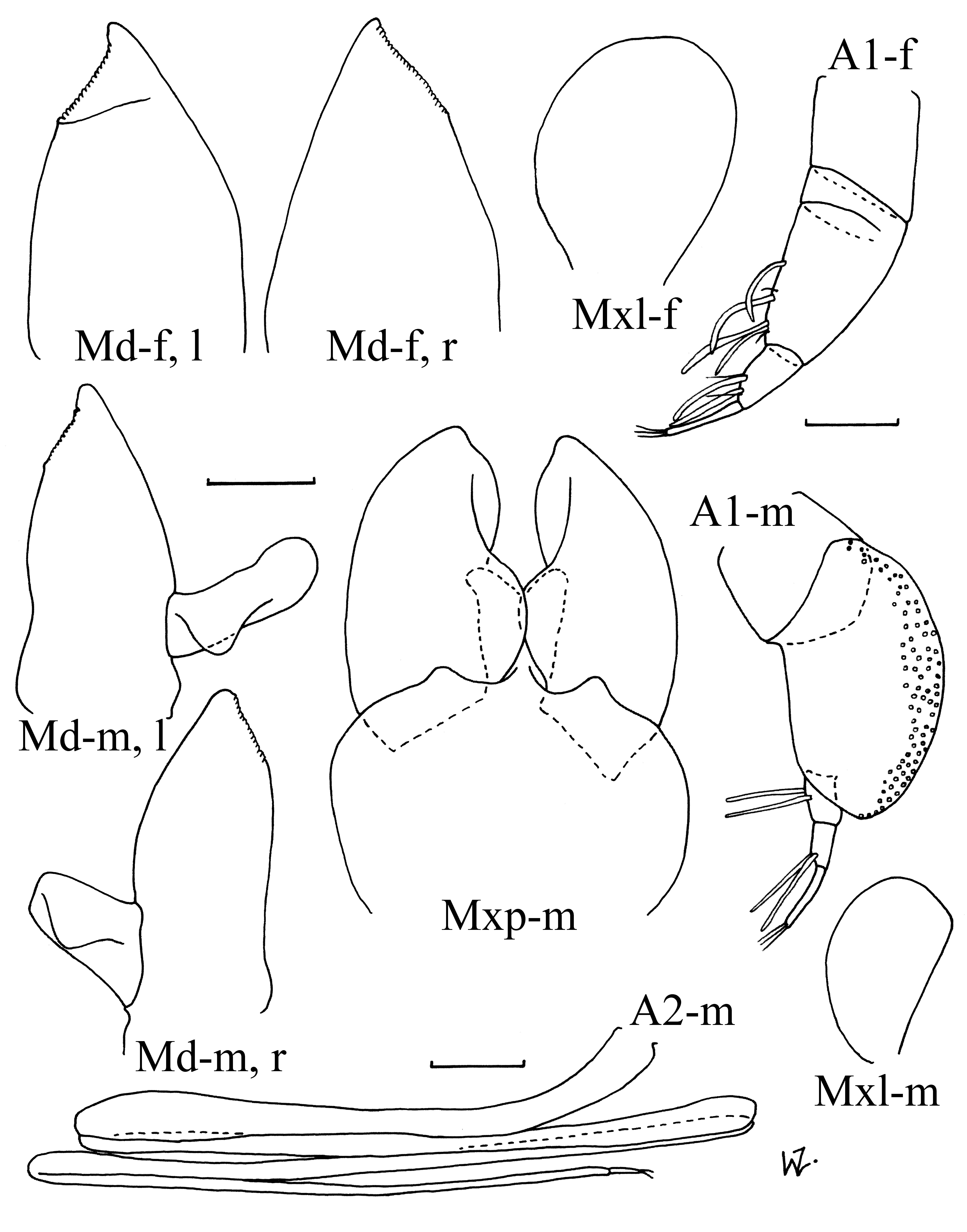
Diagnosis. Females: Sexually mature at about 7–18 mm. Head slightly longer than first 4.5 pereonites combined; with short, rounded rostrum; strongly bulged proximally with distinct neck. Eyes occupy most of head except for neck and rostrum. Pereon cylindrical, elongate, length about 1.7 x pleon. Pleonites with postero-distal corner produced into small point. Gnathopoda relatively small, barely reaching to middle of basis of P3. Gnathopod 1; basis sub-equal in length to remaining articles combined, relatively narrow; merus relatively short, about 0.2 x basis; carpus spoon-shaped, projecting under propodus to base of dactylus, produced into strong terminal tooth, sometimes armed with 1–3 strong seta on posterior margin near base of tooth-like structure together with some less strong setae; propodus slightly curved, forming weak chela with carpus, with or without slight, but distinct, tooth near postero-distal corner; dactylus slightly curved, length almost half propodus. Gnathopod 2 slightly longer and more slender than G1, similar in structure except the carpus is relatively longer and the carpal process is armed with fewer setae or is smooth, the posterior margin of the propodus is also always smooth. Pereopods 3 & 4 with relatively thin, elongate articles, similar in structure. Pereopod 3 slightly longer than P4, sub-equal in length to P5 or only 1.2 x longer; basis length about 1.3 x merus; carpus length variable, about 0.4–0.7 x merus, about 1.4–1.6 x propodus; dactylus very small. Pereopod 5 slightly longer than P6 but both are similar in structure, with relatively broad, paddle-like articles. Pereopod 5; basis with anterior margin slightly serrated, length about twice width; merus also with slightly serrated anterior margin, distally broadened, length almost half of basis, width about twothirds length; carpus with both margins slightly serrated, also distally broadened, length about 1.6–2.0 x merus, width almost half length; propodus marginally shorter than carpus; also with both margins slightly serrated, evenly narrowed distally to dactylus, width about one-third length; dactylus a very small curved nail. Pereopod 6 a little shorter than P5, similar in structure but slightly more slender; also anterior margin of basis and posterior margins of merus, carpus and propodus not serrated. Pereopod 7; basis oval-shaped, slightly longer than remaining articles combined; merus and carpus of similar length, propodus slightly longer; dactylus very small. Uropod 1; peduncle reaching beyond limit of peduncle of U2 & U3; outer ramus slightly wider than inner, length about 1.5 x inner ramus, about half of peduncle. Uropod 2; peduncle reaches limit of peduncle of U3; rami similar in length, about two-thirds peduncle. Uropod 3; peduncle very short, slightly wider than long; rami similar in length, slightly longer than 2.5 x peduncle. Telson triangular, rounded, marginally longer than wide, reaching to about half of inner ramus of U3. Margins of rami and telson finely denticulate.
Colour in life: mainly translucent, with some frequency the eyes and gut contents may also appear orange (pers. obs.).
Males: Generally like females except for the following. Antenna 1; peduncle one-articulate; callynophore large, crescent-shaped, length about 1.6 x width, with small antero-distal lobe, with aesthetascs arranged in onefield brush medially; three smaller, much narrower, articles inserted on antero-dorsal corner of callynophore. Antenna 2 of males, five-articulate; with strong zig-zagged articulation, and all but terminal article folded back on each other, under the head, extending ventro-anteriorly and ventro-posteriorly between the gnathopoda and pereopoda; first article slightly shorter than second, third slightly longer than second, fourth marginally shorter than first, terminal article very short. Pereon and pleon marginally more slender. Epimeral plates relatively much longer and deeper. Head of juveniles like that of females but more elongate (less globular) in more mature specimens. Pereopods 5 & 6 sometimes have slightly broader articles. Gills absent on pereonite 2.
Remarks. This species is readily distinguished from other members of the Oxycephalidae by the general habitus, the head shape and the structure of the gnathopoda and pereopods 5 & 6. Eye morphology clearly distinguish it from G. re b e ca e sp. nov., together with other minor characters as detailed under that species.
An examination of the material listed above, although limited, revealed considerable variation in the relative lengths of the pereopoda and pereopod articles as reflected in the above diagnosis. In addition, for both sexes, the propodus of gnathopod 1 often lacked the small distal tooth on the posterior margin; a character Bovallius (1887, 1890) used to distinguish G. spiniger from G. milneedwardsi . Sometimes only a tiny tooth was present. Also, the carpus of gnathopod 2 sometimes lacked distal setae, while in other specimens 1–3 strong setae were present ( Fig. 3 View FIGURE 3 ). These variations suggest that more than one species may be present under the current umbrella of G. milneedwardsi , although no consistent geographical variations could be determined. Clearly a more detailed analysis of specimens from a wide geographical range is required to resolve this issue, preferably utilizing molecular genotyping techniques.
A detailed examination of the remains of the holotype of G. spiniger ( Fig. 2 View FIGURE 2 ) revealed the following notable characters. The head is relatively small and rounded; the propodus of gnathopod 1 is armed with a small tooth, distally on the posterior margin; in both gnathopoda the carpus ends in a relatively long, sharp tooth; pereopod 5 is marginally longer than pereopods 3–4; pereopod 7 is very small and seems almost vestigial, and the telson is more quadrate. Generally these characters are most consistent with the specimens from the Great Barrier Reef except that in these specimens the propodus of gnathopod 1 lacks the characteristic tooth and pereopod 7 is not particularly reduced in size. One might also include Elsia indica Giles, 1890 with this group. The remainder of the specimens examined are most like the syntype of G. milneedwardsi ( Fig. 1 View FIGURE 1 ), especially in that pereopods 3 & 4 are very slender and much longer than pereopod 5. However, some specimens have a small tooth on the propodus of gnathopod 1 and additional strong setae on the carpus of both gnathopoda. One might include in this group G. adriaticus Steuer, 1911 and the specimens figured by Shoemaker (1945) from Bermuda.
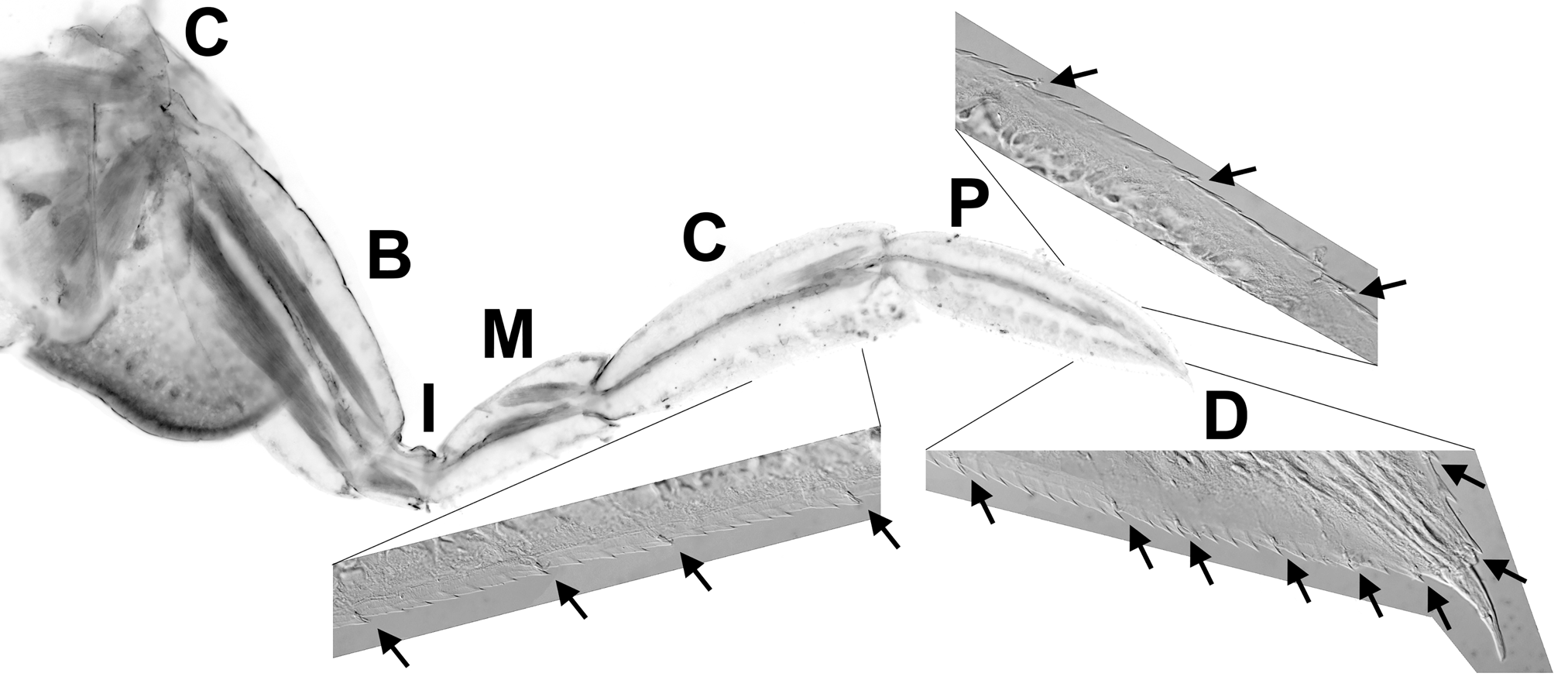
The paddle-shaped distal elements associated with pereopod 5 are also characterized by the presence of regularly distributed, innervated sensory hairs along the margins ( Fig. 6 View FIGURE 6 ). Observations on both in situ and captive animals revealed that pereopod 5 is actively ‘brushed’ against the surface of the host and may function in collecting surface debris/material. When disturbed, G. milneedwardsi will typically take refuge in the colloblast lined feeding grooves of the ctenophore rather than abandon its host ( Fig. 5 View FIGURE 5 ). Their waxy cuticle appears to be refractory to colloblast adhesive. We also note that demarsupiated G. milneedwardsi juveniles remain on the external surface of their host.
Glossocephalus milneedwardsi View in CoL has, to date, only been recorded in association with ctenophores. Namely, Deiopea kaloktenota Chun, 1879 View in CoL ( Krumbach 1911, Steuer 1911a, b), Bolinopsis vitrea Agassiz, 1860 View in CoL ( Harbison et al. 1977, 1978), Leucothea multicornis Quoy and Gaimard, 1824 View in CoL , Cestum veneris Lesueur, 1813 (Harbison et al. 1978) View in CoL , Leucothea multicornis Quoy and Gaimard, 1824 View in CoL , Beroe ovata Eschscholtz, 1829 ( Laval 1980) View in CoL , and Leucothea pulchra Matsumoto, 1988 View in CoL , and Bolinopsis rubrapunctatus Tokioka, 1964 (SAMA specimens; figs. 3–4). The latter two are new host records. We also find this species in coastal and near coastal waters commonly associated with Bolinopsis View in CoL and Mnemiopsis View in CoL ( Fig. 5 View FIGURE 5 ) in the following locations; Atlantic waters off the southern coast of Florida and several Caribbean locales along the Mesoamerican barrier reef off the coast of Belize and the Bocas del Toro archipelago off the north-eastern coast of Panama. The association of G. milneedwardsi View in CoL with Mnemiopsis View in CoL represents an additional new host record.
Fage (1960) provides some information on the biology of G. milneedwardsi View in CoL , and demonstrated that it occurred most frequently in the top 100 m, was fairly abundant down to 200 m, but very infrequent below that depth. He also found considerable differences in the stage at which females reach maturity between oceans, with females ovigerous at 7.5 mm in the Indo-Pacific region, 9.5 mm in the Mediterranean Sea and 12.5 mm in the Indian Ocean. Zeidler (1978) also found females from the Great Barrier Reef region ovigerous at about 7.0 mm.
Distribution. This is a relatively common nominal species, apparently widely distributed, mainly in the tropical regions of the world’s oceans, including the Mediterranean Sea and Red Sea. Historical distribution accounts suggest common occurrence in the Indian Ocean and less common in the Atlantic and Pacific Oceans ( Fage 1960). However, recent observations along the coast of Florida, the Mesoamerican barrier reef ( Belize), and the eastern Panamanian coast found G. milneedwardsi to be a common hyperiid species, especially in the presence of lobate ctenophores. Given their close affiliation with lobate ctenophores it is likely that demographic distributions of G. milneedwardsi fluctuate concomitant with the ephemeral boom and bust population structure associated with its host.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Glossocephalus milneedwardsi Bovallius, 1887
| Zeidler, Wolfgang & Browne, William E. 2015 |
Glossocephalus milneedwardsii
| Brusca 1981: 12 |
Glossocephalus milneedwardsi
| Hurt 2013: 31 |
| Gasca 2012: 126 |
| Valencia 2012: 1493 |
| Mori 2010: 10 |
| Gasca 2009: 89 |
| Gasca 2009: 66 |
| Gasca 2009: 218 |
| Lavaniegos 2009: 142 |
| Valencia 2009: 268 |
| Gasca 2008: 569 |
| Browne 2007: 819 |
| Madrigal 2007: 156 |
| Gasca 2007: 118 |
| Brusca 2005: 152 |
| Gates 2003: 328 |
| Escobar-Briones 2002: 367 |
| Gasca 2001: 496 |
| Lowry 2000: 327 |
| Nair 1995: 19 |
| Zeidler 1984: 298 |
| Vinogradov 1982: 427 |
| Thurston 1976: 438 |
| Tashiro 1972: 12 |
Glossocephalus milne
| Harbison 1977: 479 |
| Hure 1969: 603 |
Glossocephalus
| Pirlot 1939: 55 |
| Pirlot 1939: 78 |
| Pirlot 1938: 43 |
Glossocephalus milne
| Lin 1996: 231 |
| Lin 1995: 123 |
| Shih 1995: 200 |
| Lin 1994: 114 |
| Laval 1980: 20 |
| Zeidler 1978: 34 |
| Bowman 1973: 51 |
| Yoo 1971: 43 |
| Dick 1970: 42 |
| Pillai 1966: 186 |
| Fage 1960: 83 |
| Shoemaker 1945: 253 |
| Barnard 1940: 485 |
| Barnard 1937: 193 |
| Barnard 1931: 131 |
| Spandl 1927: 196 |
Glossocephalus
| Cecchini 1929: 485 |
| Stephensen 1925: 202 |
| Colosi 1918: 221 |
Glossocephalus adriaticus
| Steuer 1911: 352 |
| Steuer 1911: 682 |
Elsia indica
| Walker 1904: 237 |
| Giles 1890: 250 |
Glossocephalus Milne-Edwardsi Bovallius, 1887 : 35
| Chevreux 1935: 200 |
| Chevreux 1925: 433 |
| Chevreux 1913: 11 |
| Bovallius 1890: 105 |
| Bovallius 1887: 35 |
Glossocephalus spiniger
| Spandl 1927: 196 |
| Steuer 1911: 352 |
| Steuer 1911: 683 |
| Bovallius 1890: 105 |
| Bovallius 1887: 35 |