Nesodiprion japonicus ( Marlatt, 1898 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.209562 |
|
DOI |
https://doi.org/10.5281/zenodo.3507224 |
|
persistent identifier |
https://treatment.plazi.org/id/D26C4539-FFBE-FFA6-7AEE-5A62FBB14948 |
|
treatment provided by |
Plazi |
|
scientific name |
Nesodiprion japonicus ( Marlatt, 1898 ) |
| status |
|
Nesodiprion japonicus ( Marlatt, 1898)
Figs. 1A–F View FIGURES 1 A – F ; 3A–D; 4A–D; 5A–C; 6A, B; 7A, B; 8A–F; 9A, B; 10A–L; 13A–E; 14A–D
Lophyrus japonicus Marlatt, 1898: 506 .
Nesodiprion japonica: Rohwer (1910: 104) , Takeuchi (1940: 190).
Nesodiprion japonicus: Smith (1974: 216) , Abe & Togashi (1989: 545), Wei et al. (2006: 551), Taeger et al. (2010: 210).
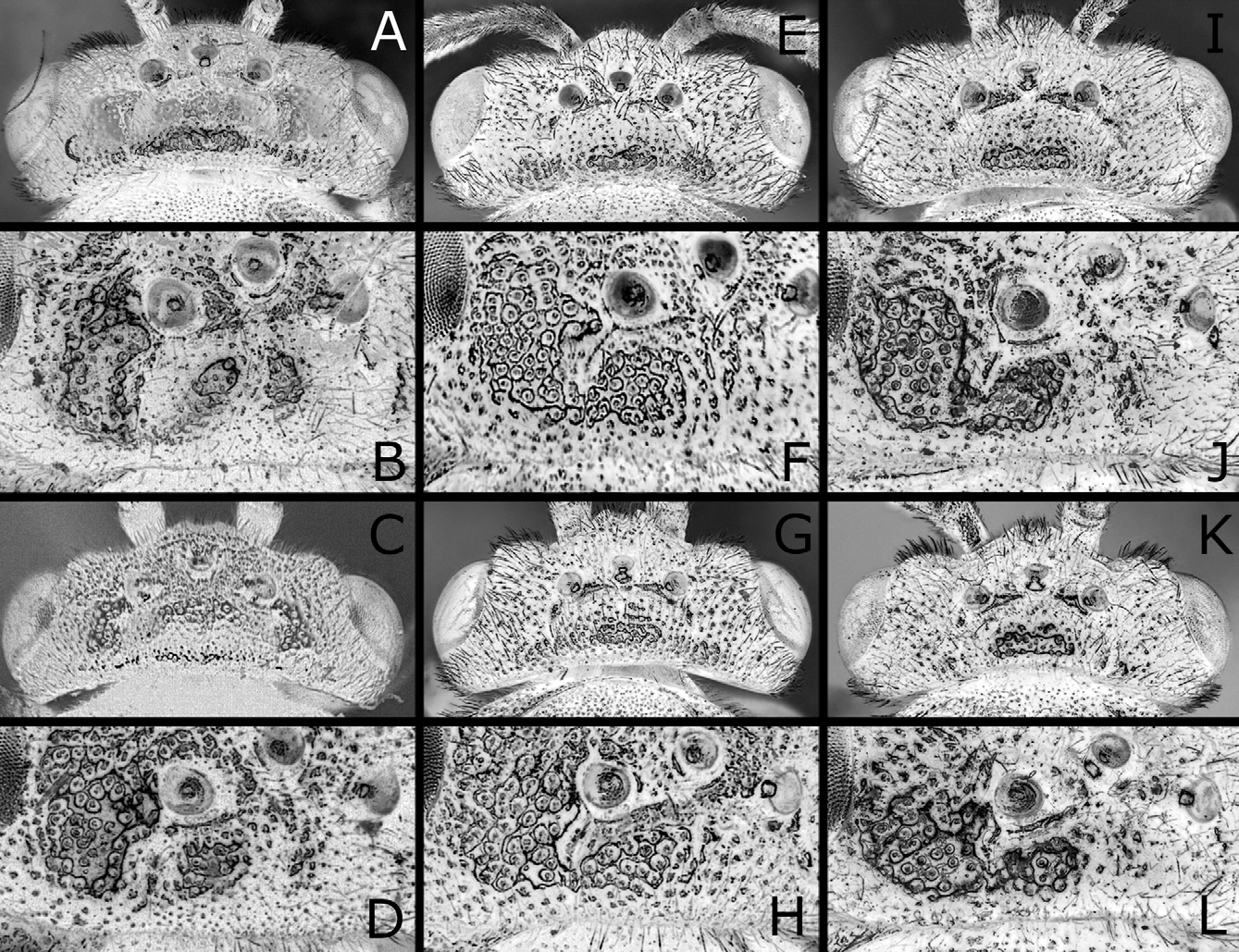
Female. Length 7–9 mm. Black, shiny, without metallic reflection, sometimes faintly metallic violet on abdomen ( Figs. 1A–D View FIGURES 1 A – F ). Ventral half of clypeus and labrum often yellowish to brownish. Mandible apically reddish. Basal two to three antennomeres often slightly brownish. Palpi yellow to pale brown. Pronotum yellowish white to brown on posterior corner. Postspiracular sclerite white or pale brown to black. Median mesoscutal lobe sometimes yellowish posterolaterally. Mesoscutellum predominantly or mostly yellowish white. Legs white to yellow on apices of coxae to trochantelli, apices of femora, fore and middle tibiae, wide basal part of hind tibia and tarsi; hind tibia dark brown to black on apical fourth, usually slightly and narrowly pale at apex; fore and middle tibiae and tarsi each apically slightly darkened; spurs brown. Wings hyaline; veins largely brown to black; in fore wing, vein C except for apex yellow, vein R1 basal to stigma partly yellowish, and stigma somewhat pale apically. Seventh and eighth abdominal terga each laterally with yellowish white spot ( Fig. 1D View FIGURES 1 A – F ), sometimes sixth abdominal tergum laterally narrowly yellowish white. Cercus black. Setae largely whitish.
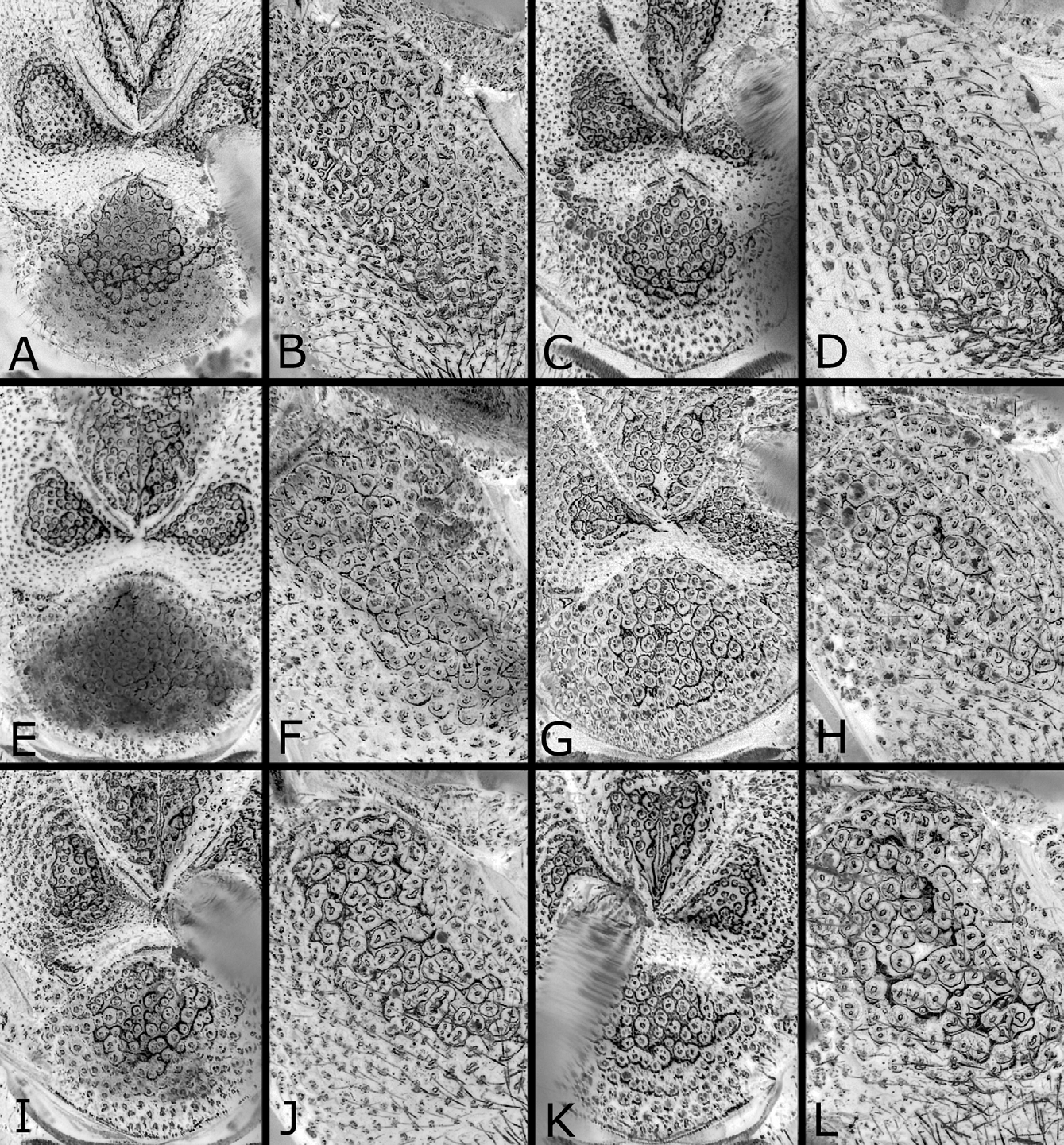
Head and thorax shiny, with punctures predominantly distinct and dense; on dorsum of head ( Figs. 3B, D View FIGURES 3 A – L ), punctures fine and predominantly separated, and interspaces predominantly wider than punctures; on mesoscutum ( Fig. 4A View FIGURES 4 A – L ), punctures fine, those on posterior part of median lobe somewhat vague, mostly separated and about as large as those on lateral lobe, and interspaces on posterior part of median lobe mostly wider than punctures; interspaces on center of mesoscutellum largely not linear-shaped; on mesepisternum ( Fig. 4B View FIGURES 4 A – L ), punctures predominantly contiguous, but interspaces predominantly not linear-shaped. Clypeus with wide ventromedial part nearly smooth or faintly punctured. Labrum smooth. Abdomen shiny; first tergum ( Fig. 5A View FIGURES 5 A – I ) punctured on narrow medial part to medial third, or not; second to fifth terga dorsally nearly smooth; sixth tergum to apex faintly punctured; ventral surface somewhat dull and weakly punctured.
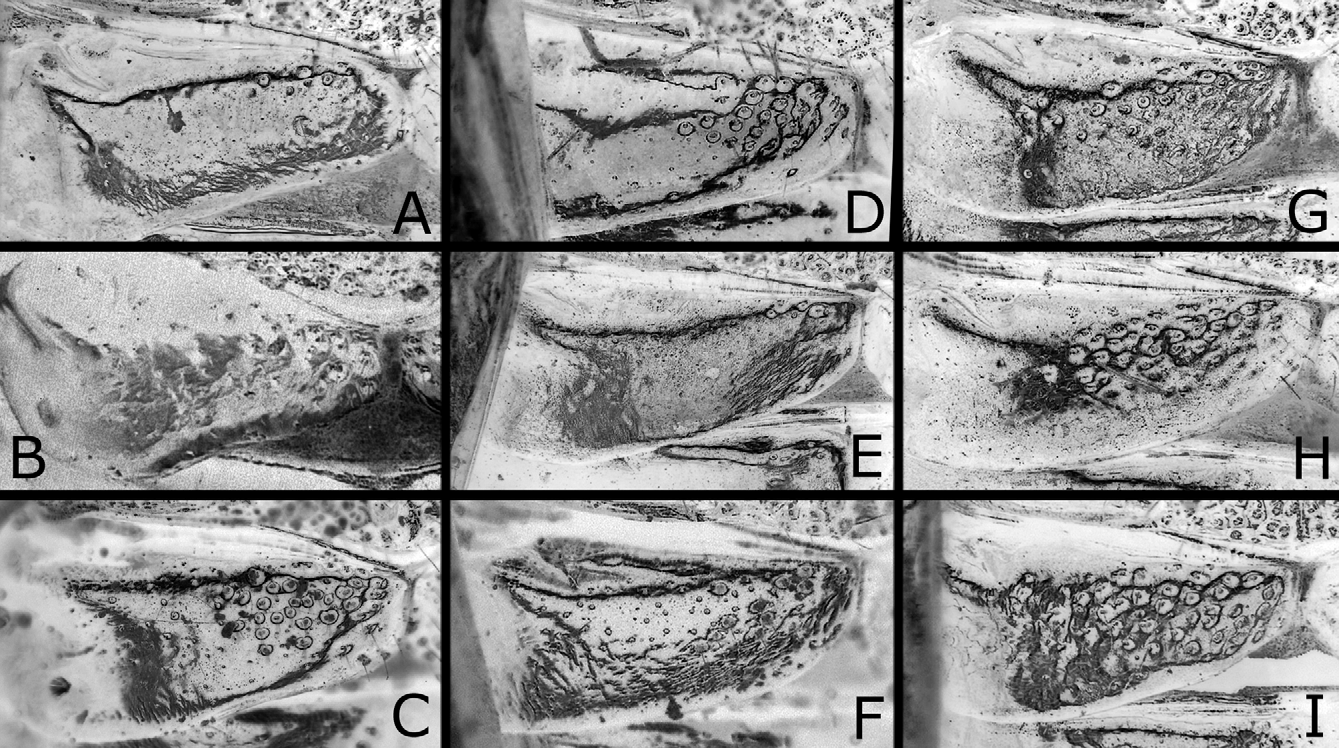
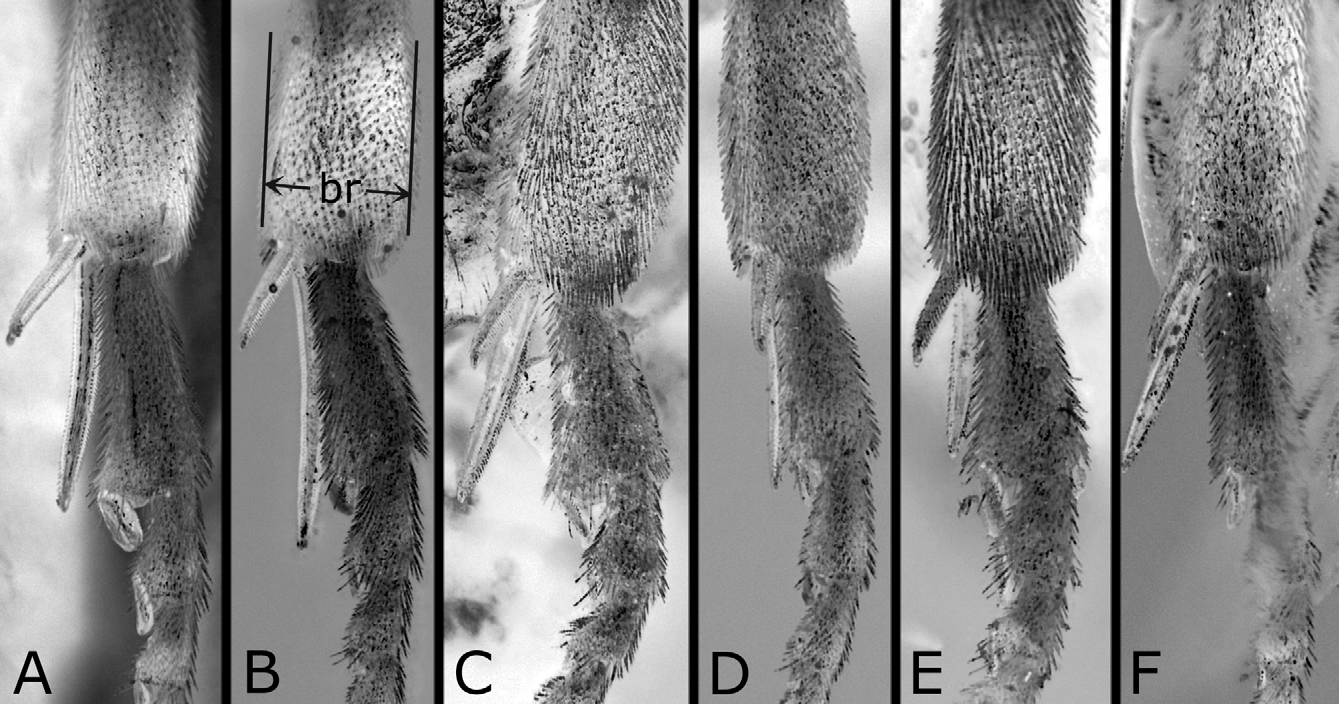
Postocellar area weakly or moderately convex ( Figs. 3A, B View FIGURES 3 A – L ), with lateral furrow distinct on anterior two-thirds and anterior furrow medially blunt widely, and often with weak median furrow. Distances between eye and hind ocellus, between hind ocelli, and between hind ocellus and posterior margin of head 0.9–1.1: 1.0: 0.9–1.3; distances between eye and hind ocellus and between hind ocellus and posterior margin of head 0.8–1.0: 1.0. Distance between torulus and eye 1.5–1.8 × distance between toruli. Width of malar space 0.1–0.4 × width of front ocellus, 0.3–0.6 × length of second antennomere. Clypeus with ventral margin roundly concave. Antenna ( Fig. 6A View FIGURES 6 A – E ) with 21–23 antennomeres; length of second antennomere 0.5–0.8 × width of front ocellus; length of ramus of third antennomere 2.7–4.1 × length of third antennomere. Mesoscutellum ( Fig. 4A View FIGURES 4 A – L ) dorsally flattened widely, rarely slightly convex, sometimes with weak median furrow. Hind leg ( Fig. 7A View FIGURES 7 A – F ) with length of inner tibial spur 1.1–1.4 × length of first tarsomere (exclusive of pulvillar pad), 1.7–2.1 × breadth of tibia; length of first tarsomere 1.3–1.8 × breadth of tibia; second and third tarsomeres combined 0.8–1.0 × first in length.
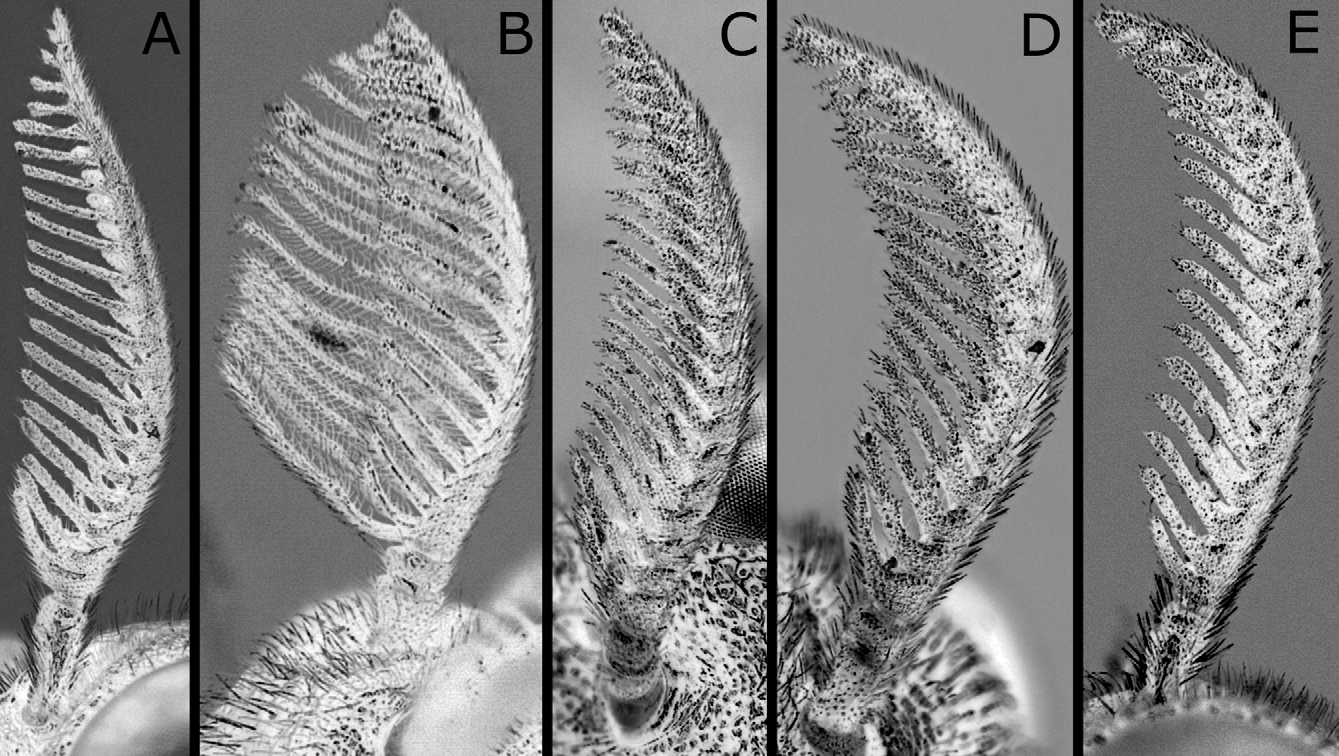
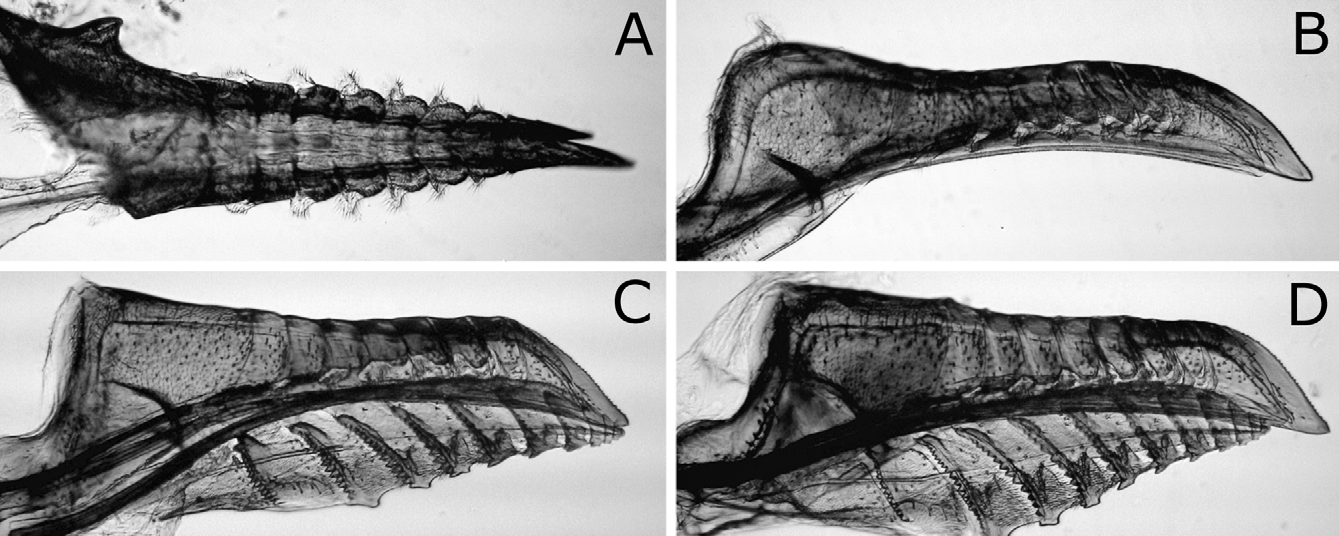
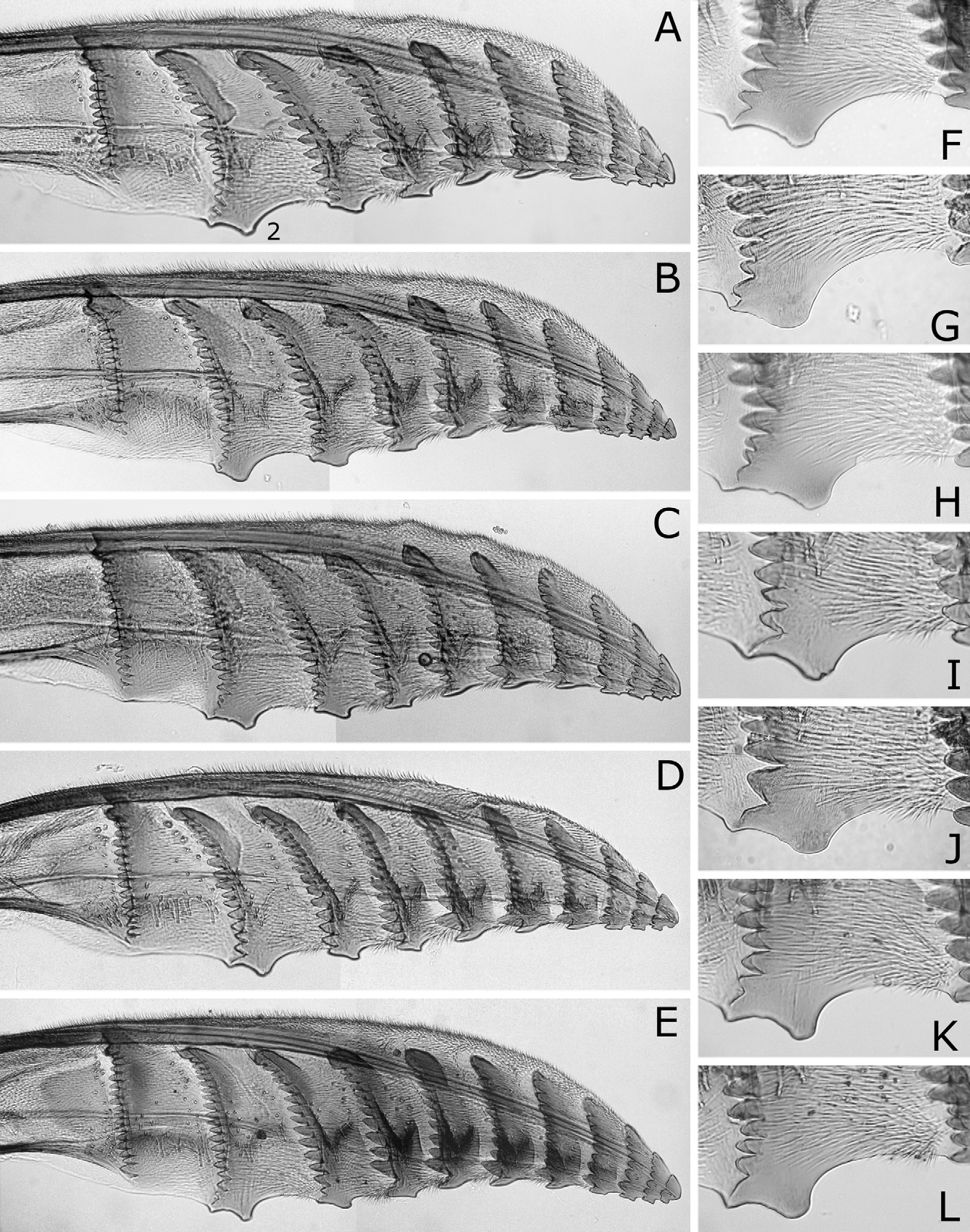
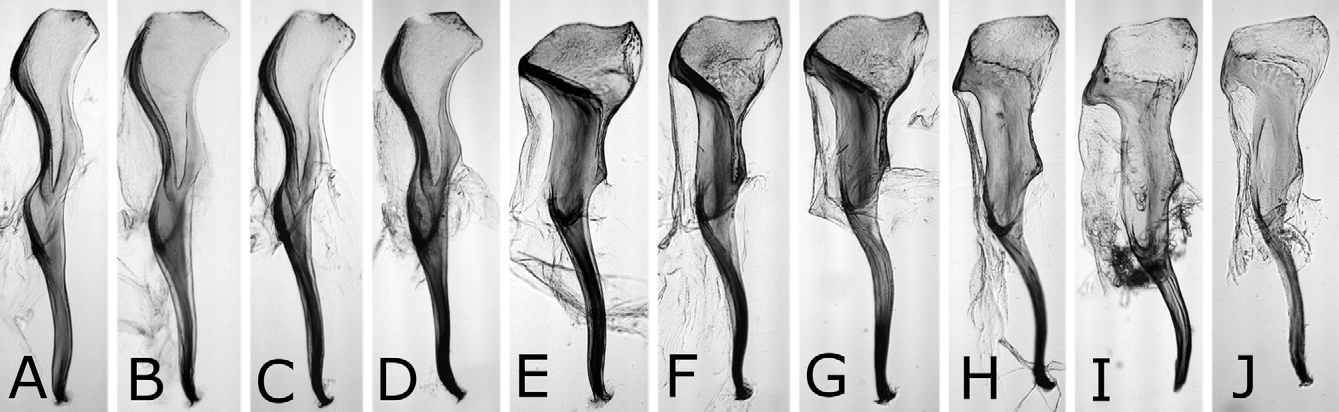
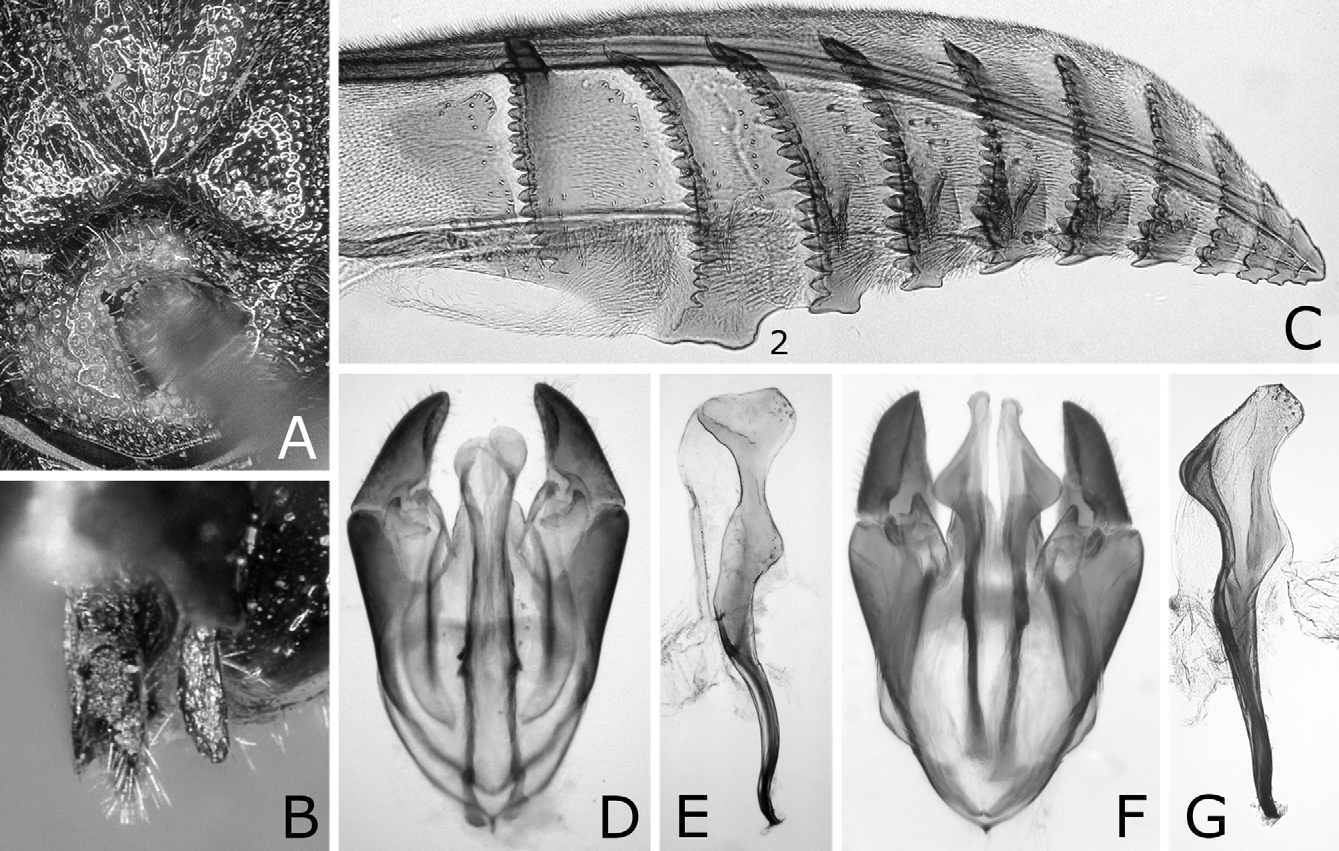
Sawsheath in dorsal view narrow, not tapering apically, with inner margin concave, and apex much wider than cercus (Figs. 8A, D), in lateral view slightly roundly convex apically (Figs. 8B, E), and in posterior view with scopa vertically elongate (Figs. 8C, F). Lance in lateral view with dorsal margin slightly concave at middle ( Fig. 9B View FIGURES 9 A – D ); apices of lances asymmetrical, either left or right one longer than another ( Fig. 9A View FIGURES 9 A – D ). Lancet ( Figs. 10A–E View FIGURES 10 A – L ) with 10–11 annuli, widest at second annulus, and length from apex to ventral end of basal row of spines 2.7–2.8 × maximum width; spines relatively long; border of first and second annuli ventrally weakly but distinctly convex angularly; serrula of second annulus ( Figs. 10F–L View FIGURES 10 A – L ) apically narrowly truncate, rarely nearly rounded, with anterior slope much shorter than posterior slope; serrula of third annulus with anterior slope slightly concave.
Male [condition of lectotype in brackets]. Length 6.5–8.5 [7] mm. Coloration as in female except for mesoscutum, mesoscutellum and abdomen always entirely black ( Figs. 1E, F View FIGURES 1 A – F ). [Ventral half of clypeus dark brown; labrum brown; basal two antennomeres very slightly pale; posterior corner of pronotum yellowish white; postspiracular sclerite brown.]
Structure as in female except for usual sexual differences. Punctures more distinct ( Figs. 3D View FIGURES 3 A – L , 4C, D View FIGURES 4 A – L ); on dorsum of head, punctures somewhat larger, predominantly separated or contiguous [contiguous], and interspaces predominantly narrower or wider than punctures [narrower]; on mesepisternum, interspaces partly or moderately linear-shaped ( Fig. 4D View FIGURES 4 A – L ), rarely mostly so as in Fig. 4H View FIGURES 4 A – L [moderately]. [First abdominal tergum with punctures on about medial third ( Fig. 5B View FIGURES 5 A – I ).] Postocellar area with lateral furrow distinct on anterior half ( Fig. 3D View FIGURES 3 A – L ); weak median furrow rarely present [present]. Distances between eye and hind ocellus, between hind ocelli, and between hind ocellus and posterior margin of head 0.8–1.1: 1.0: 0.8–1.0 [0.8: 1.0: 0.8]; distances between eye and hind ocellus and between hind ocellus and posterior margin of head 1.0–1.2: 1.0 [1.1: 1.0]. Distance between torulus and eye 1.3–1.6 [1.5] × distance between toruli. Width of malar space 0.4–0.6 [0.6] × width of front ocellus, 0.6–1.1 [0.8] × length of second antennomere. Antenna ( Fig. 6B View FIGURES 6 A – E ) with 21–23 [21] antennomeres, 1.0–1.1 [1.0] × as long as head width; length of second antennomere 0.4–0.8 [0.8] × width of front ocellus; ramus of third antennomere very long. Hind leg ( Fig. 7B View FIGURES 7 A – F ) with length of inner tibial spur 1.1–1.4 [1.1] × length of first tarsomere, 1.7–2.2 [2.0] × breadth of tibia, length of first tarsomere 1.3–1.8 [1.8] × breadth of tibia, and second and third tarsomeres combined 0.8–1.0 [0.9] × first in length. [Mesoscutellum dorsally slightly convex, without median furrow ( Fig. 4C View FIGURES 4 A – L ).]
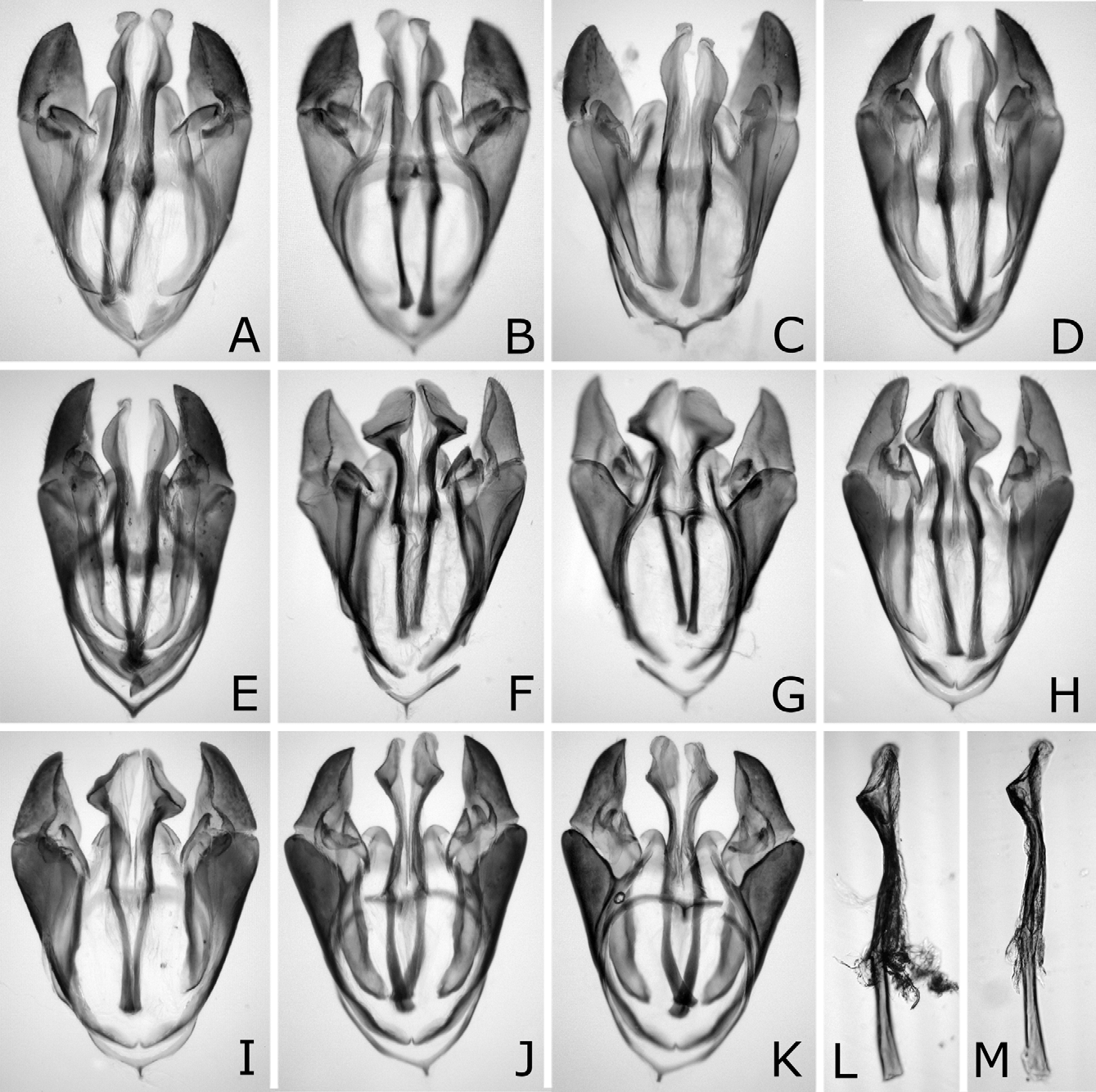
Genitalia with valviceps in dorsal view laterally convex weakly and roundly on apical half, and laterally convex narrowly at apex ( Figs. 13A, C–E View FIGURES 13 A – M ), and in lateral view sinuate and gradually widened toward apex, with dorsal margin weakly convex near apex and ventral margin angularly convex near apex ( Figs. 14A–D View FIGURES 14 A – J ).
Type material examined. Lectotype (here designated): 3, with “ 25. 7. 14, [Gifu] (in Japanese letters), 3” written on cardboard and a label “ Japan, Mitsukuri” ( USNM). The lectotype was originally mounted on a pale brown cardboard as in Fig. 1A View FIGURES 1 A – F as stated by Marlatt (1898), but it was removed and directly pinned for this study ( Figs. 1E, F View FIGURES 1 A – F ). Paralectotypes ( USNM): 13, labeled as in lectotype; 1Ƥ, do., but with additional label “Ƥ Type No. 3839, U.S. N.M.”; 13, labeled as in lectotype, with additional labels “HymSlide 198-201”, “trophi mounted”, “genitalia mounted” and “wing mounted”; 23, labeled as in lectotype, but “ 25. 7. 15 ” on cardboard; 23, labeled as in lectotype, but “ 25. 7. 20 ” on cardboard; 13, labeled as in lectotype, but “23. 0. 0 0, [Gifu, near pine, sawfly] (in Japanese letters)” on cardboard and with additional label “3 Type No. 3839, U.S. N.M.”.
Marlatt (1898) described this species from two females and nine males from “Gifu”, but did not designate a holotype. In the preface, he wrote “based on material presented by Dr. K. Mitsukuri” and “All of the specimens are mounted on large flat cards, with wings and legs beautifully spread”. We located one female and eight male syntypes in USNM. They are mounted on or bear the same cardboards (the data written are partly different, except for [Gifu], as stated above; one male has been already removed from the cardboard), and bear the same labels “ Japan, Mitsukuri”. Therefore, they are safely considered the syntypes, although only one female and one male have the type labels. We designate a male syntype without the type label as the lectotype, because the female of N. japonicus is often indistinguishable from that of N. kagaensis (see under Comparative notes), and the male syntype with the type label is not in good condition (most of the legs are missing).
Other material examined. JAPAN ―Hokkaido: 2Ƥ53, Mori, VIII. 1971, Host Pinus strobus, K. Kamijo ( HFRI, NSMT). Honshu―Fukushima Pref.: 13, “Wakamatsu”, 30. V. 1951, Y. K. (KU). Kanagawa Pref.: 7Ƥ43, Zu, 28. VII. 1973, Y. Hasegawa ( NSMT, SDEI). Gifu Pref.: 1Ƥ33, “Katayama”, 2-5. IX. 1920, Takeuchi ( OPU). Ishikawa Pref.: 13, Kanazawa, Kakuma, 14. VII. 1996, M. Eguchi ( NSMT). Kyoto Pref.: 13, Kyoto, 20. IV. 1927, Takeuchi ( OPU); 13, do., 10. IX. 1932 ( OPU); 13, do., 25. X. 1932 ( OPU); 4Ƥ13, Kyoto, Kitashirakawa, VII. 1942, M. Tokunaga ( OPU). Hyogo Pref.: 13, Sasayama, 3. V. 1961, T. Naito (KU); 33, Rokko, 12. X. 1977, N. H. (KU). Shikoku― Kochi Pref.: 3Ƥ, Kochi, 16. VI. 1952, Takeuchi, J. Wada ( OPU). Satsunan Islands: 13, Yakushima, Hinokuni, 27-30. III. 1971, K. Yamagishi (KU); 1Ƥ 13 in copula, Amami-oshima, Naze, 19. V. 1955, Takeuchi, S. Ito ( OPU). Ryukyu Islands: 23, Okinawa-jima, Kunigami, 18. VI. 2003, G. A. Show ( NSMT); 23, do., Naha, 1. III. 1928, Host Pinus luchuensis, H. Yashiro ( OPU) (cited by Takeuchi, 1940); 13, Iriomote-jima, Ohara, 22. XI. 1960, K. Yasumatsu (KU). KOREA ―Kangwan-do: 13, Tokchom-kogae, 510 m, nr. Chuncheon, 4. VI. 1991, A. Shinohara ( NSMT); 1Ƥ, Chuncheon, Nam-myeon, Hudong-li, 17. VIII.–5. IX. 2003, Tripotin ( USNM); 1Ƥ23, do., but Magog-li, 70 m, 11. VII.–7. VIII. 2004 ( USNM, NSMT); 1Ƥ, do., but Seokdong, Pohyeonsa, 31. VII.–28. VIII. 2005, Tripotin ( NSMT). Jeollabuk-do: 13, Iksan, Geumma-myeon, Miluk-san, 12–18. VII. 2004, C. L. Young ( USNM). TAIWAN: 5Ƥ53, “ Formosa: 1973, ex pine” ( USNM, NSMT); 2Ƥ13, Taipei, 3. VIII. 1928, K. Sibata ( OPU). USA: 1Ƥ13, “on Pine from Japan, from Alex. Craw., S. Francisco, Calif, April 3, 1902 ” ( USNM).
Distribution. Japan: Hokkaido ( Yogo 1965), Honshu (type locality), Shikoku ( Matsushita 1943; Togashi 1974), Kyushu ( Yano 1916), Yaku-shima (new record), Amami-oshima ( Sato 1981), Okinawa-jima ( Takeuchi 1940) and Iriomote-jima ( Abe & Togashi 1989); Korea ( Kim 1963); Taiwan ( Mitono 1936). Although we have not examined specimens of the authors cited above except for those of Okinawa-jima by Takeuchi (1940), we were able to examine other specimens from these areas except for Kyushu.
Rohwer (1910) reported that this species “came to the port of San Francisco on a Japanese pine in 1902” and “has been introduced into United States through San Francisco, California”, and Takeuchi (1940) wrote that this species was “introduced into North America ”. Rohwer’s report is based on a female and a male labeled “on Pine from Japan, from Alex. Craw., S. Francisco, Calif, April 3, 1902 ” (USNM). These are quarantine interceptions at U. S. ports-of-entry and do not indicate establishment. Nesodiprion japonicus has never been collected in North America.
Host plants. Pinaceae : Cedrus deodara ( Okutani 1959, 1967); Larix kaempferi ( Yano 1916; Okutani 1959, 1967); Pinus densiflora ( Yano, 1916; Okutani 1967), P. koraiensis ( Sato 1981) , P. leiophylla ( Furuno 1976) , P. luchuensis ( Matsushita 1943) , P. massoniana ( Mitono 1936) , P. palustris ( Okutani 1967) , P. radiata ( Sato 1981) , P. strobus ( Yogo 1965; Okutani 1967), P. taeda ( Okutani 1967) , P. thunbergii ( Yano 1916) , P. wallichiana ( Furuno 1976) , “slash pine ( P. caribaea )” ( Yie et al. 1966a). We have only examined specimens reared on P. luchuensis and P. strobus . Other host plants need confirmation (see under Remarks).
Life history. Adults have been collected in the field from late April to late October in Kyoto Prefecture, Honshu and from early March to late November in the Ryukyu Islands. The sawfly is probably multivoltine in the temperate and subtropical regions. Previous studies need confirmation (see under Remarks).
Comparative notes. The three species treated here, N. japonicus , N. biremis and N. orientalis , and one Japanese species, N. kagaensis , are similar to each other in having the following combination of characters: Head, thorax and abdomen mostly black; legs black, with trochanters and their adjacent areas white to brown, distinctly pale, and apices of femora, wide basal area of hind tibia and most of tarsi white to yellow; distances between eye and hind ocellus and between hind ocelli 0.8–1.3: 1.0; malar space narrower than front ocellus; hind tarsus with second and third tarsomeres combined 0.8–1.1 × first in length; in female, seventh and eighth abdominal terga usually each with white to yellowish brown lateral spot, third antennomere with ramus more than 1.5 × length of third antennomere, and sawsheath in dorsal view narrow and apically incised. Four Chinese species, N. yananicus , N. zhejiangensis , N. huanglongshanicus and N. degenicus , are probably similar to the above four species, because N. yananicus , N. zhejiangensis and N. huanglongshanicus were described as resembling N. japonicus and N. degenicus as closely allied to N. yananicus ; however, their characters were insufficiently detailed (see Xiao et al. 1981, 1984, 1985, 1992; Zhu et al. 1983), and we have not examined any specimens safely identifiable with these species except for the lancet and penis valve of N. degenicus .
Nesodiprion japonicus is separated from N. biremis and N. orientalis by the fine punctures on the posterior part of the mesoscutum ( Figs. 4A, C View FIGURES 4 A – L ), and punctures on the median mesoscutal lobe about as large as those on the lateral mesoscutal lobe (in N. biremis and N. orientalis , the punctures large ( Figs. 4E, G, I, K View FIGURES 4 A – L ), and the punctures on the median lobe larger than those on the lateral lobe). For more comparisons, see under the latter two species.
Nesodiprion japonicus is quite similar to N. kagaensis , and the main distinguishing characters are in the penis valves. The valviceps in dorsal view is laterally weakly and roundly convex and in lateral view is dorsally convex near the apex in N. japonicus ( Figs. 13A–E View FIGURES 13 A – M , 14A–D View FIGURES 14 A – J ), while in dorsal view more strongly and nearly angularly so and in lateral view dorsally almost straight near the apex in N. kagaensis ( Figs. 16F, G View FIGURES 16 A – G ). Their females are often not distinguishable except when females are safely or tentatively identifiable with either species, namely collected together with certain males in copulation, whose progeny were reared, or considered as being collected together with certain males. In such females, the mesoscutellum is always predominantly pale in N. japonicus , while predominantly pale to dark or entirely dark in N. kagaensis , and the seventh and eighth abdominal terga each are always laterally distinctly pale in N. japonicus , while pale or absent in N. kagaensis . The malar space width is 0.1–0.4 × the front ocellus width in N. japonicus , whereas 0.3–0.7 × in N. kagaensis . Thus, pale females having a very narrow malar space (0.1–0.2 × the front ocellus in width) are N. japonicus , pale females having a moderate malar space (0.3–0.4 ×) are not identifiable, and pale females having a wide malar space (0.5 × or more) and dark females are N. kagaensis . The holotype of N. kagaensis (examined, deposited in NSMT) is a female, with the mesoscutellum mostly black, the abdomen entirely black, and the malar space 0.6 × the width of the front ocellus ( Togashi 1998). Nesodiprion kagaensis will be detailed in a separate paper.
According to the original descriptions of N. yananicus , N. zhejiangensis , N. huanglongshanicus and N. degenicus ( Xiao et al. 1981, 1984, 1985), N. yananicus has the head and thorax shiny, the head, pronotum and mesoscutellum with coarse punctures, and the median and lateral mesoscutal lobes with small and uniform punctures; N. zhejiangensis has the head and thorax moderately shiny with dense and coarse punctures; N. huanglongshanicus has the head and thorax moderately shiny, the head, pronotum and mesoscutellum with coarse and somewhat sparse punctures, and the median and lateral mesoscutal lobes with somewhat small and uniform punctures; and N. degenicus has the head and thorax with “bluish purplish reflection” and dense punctures and the mesoscutellum and “[front-lateral mesoscutal lobe]” (in Chinese) with somewhat sparse punctures. Therefore, N. japonicus is apparently similar to N. yananicus in lacking metallic reflection on the head and thorax and the fine punctures on the mesoscutum. Concerning the body color of N. yananicus, Xiao et al. (1981) only wrote that the body is black, but the seventh and eighth abdominal terga are each laterally with a pale yellow white mark, and did not refer to color of the pronotum, mesoscutellum and legs. Later, Xiao et al. (1984) wrote that the female and male pronotums are black. We suppose the color of N. yananicus is generally similar to that of N. japonicus , because the authors wrote N. yananicus resembling N. japonicus , while they did not use color for separating these two species (cf. Xiao et al. 1981). The males are easily separable by the penis valve. The valviceps in lateral view is sinuate and gradually widened toward the apex in N. japonicus ( Figs. 14A–D View FIGURES 14 A – J ), whereas the valviceps is distinctly constricted at the apical third in N. yananicus as in Fig. 16E View FIGURES 16 A – G ( Xiao et al. 1981: fig. 1; Xiao et al. 1985: fig. 15). In the female, the lancet of N. japonicus is wide, with the length from the apex to the ventral end of the basal row of spines 2.7–2.8 × the maximum width ( Figs. 10A–E View FIGURES 10 A – L ), while the lancet of N. yananicus is narrow, with the length 3.5–3.7 × the maximum width ( Xiao et al. 1981: fig. 4; Xiao et al. 1985: fig. 35). Xiao et al. (1981) distinguished the female of N. yananicus from that of N. japonicus by the smaller serrulae of the second and third annuli and the parallel third “annulus” (= probably the row of spines) and fourth “annulus”. However, these serrulae are somewhat variable in size, and these rows are almost parallel in N. japonicus ( Figs. 10A–E View FIGURES 10 A – L ).
Remarks. “ Nesodiprion japonicus ” has long been known as an important pest of pines in Japan, Korea and Taiwan. Its pest status has been recognized for more than 100 years in Japan ( Matsumura 1899, “ Lophyrus japonicus ”; Sasaki 1900, “ Lophyrus pallida!”). Many studies on the sawfly have been accumulated in these countries (e.g., Matsumura 1899; Sasaki 1900; Niijima 1913; Mitono 1936; Matsushita 1943; Okutani 1959; Inoue 1960; Kim 1963; Yie et al. 1966a, 1966b, 1967; Yie & Hsu 1967; Sato 1981; Lee & Kim 1994; Lee & Chung 1997). However, it is probable that previous studies confused two or more species. Sato (1981) gave the most detailed study on biology and damage of “ N. japonica ” in Japan, based on a population severely infesting manmade forests of Pinus strobus in Iwate Prefecture, Honshu from 1966 to 1974. We examined specimens from his study kept in FFPRIH and HFRI, and have recognized that all the males (n=10) are N. kagaensis (the females of these two species are often indistinguishable as stated above). Okutani (1959) described the larva of “ N. japonica ” in detail, and all males (n=2) of Nesodiprion reared before 1959 in KU where his material has been deposited are N. kagaensis . In Korea, two species, N. japonicus and N. biremis , have been recorded ( Kim 1963 and Zombori 1978, respectively). In Taiwan, we have found two Nesodiprion species associated with Pinus , N. japonicus and N. sp. (? huanglongshanicus ) (see under Materials and Methods), although the former has been known as the only representative of the genus in the area. Reliable characters to separate these four species have not been detailed until this study. Previous studies on “ N. japonicus ” in these countries must be confirmed.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nesodiprion japonicus ( Marlatt, 1898 )
| Hara, Hideho & Smith, David R. 2012 |
Nesodiprion japonicus:
| Taeger 2010: 210 |
| Wei 2006: 551 |
| Abe 1989: 545 |
| Smith 1974: 216 |
Nesodiprion japonica:
| Takeuchi 1940: 190 |
| Rohwer 1910: 104 |
Lophyrus japonicus
| Marlatt 1898: 506 |