Cryptoprocta ferox, Bennett, 1833
|
publication ID |
https://doi.org/ 10.5281/zenodo.5676533 |
|
DOI |
https://doi.org/10.5281/zenodo.5698345 |
|
persistent identifier |
https://treatment.plazi.org/id/D51587EF-FFEA-9A30-F0C4-1DFDFCB9F762 |
|
treatment provided by |
Conny |
|
scientific name |
Cryptoprocta ferox |
| status |
|
1. View On
Fosa
Cryptoprocta ferox View in CoL
French: Fossa / German: Fossa / Spanish: Fosa
Other common names: Fossa, Tratraka, Kintsala
Taxonomy. Cryptoprocta ferox Bennett, 1833 View in CoL ,
Madagascar.
The interpretation of the systematic position of C. ferox has varied over the years, because these animals possess characteristics seemingly diagnostic of the families Herpestidae , Viverridae , and Felidae . The ambiguities in understanding the evolution of this genus are now partially resolved. Apparently, all native Madagascar Carnivores represent a monophyletic endemic lineage derived from a single colonization of the island. They are now placed in the family Eupleridae . Cryptoprocta typicus is a synonym of C. ferox. A notably larger form of this genus, C. spelea, was identified from bone remains recovered from Holocene sites. At some of these sites remains of both C. spelea and C. ferox have been found, but due to a lack ofstratigraphic control it is unknown if they were temporally sympatric. The name C. antamba applied to some subfossil remains appears to be a teratological C. spelea. In different parts of Madagascar, people living in the countryside note the presence of two forms of Cryptoprocta —fosa mainty or “black Cryptoprocta’ and fosa mena or “reddish Cryptoprocta ”; the red form is said to be smaller. In the extreme south-west there are reports of a whitish morphotype. It remains unclear whether the differentiation of these formsis simply folklore or is associated with some general pattern of variation (age, sex, geographic) in extant C. ferox. Monotypic.
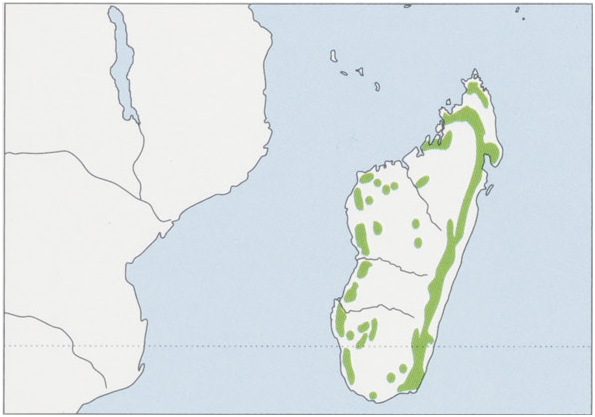
Distribution. Madagascar. View Figure
Descriptive notes. Head-body 70-80 cm, tail 65-70 cm, hindfoot 12-12. 8 cm; weight 6.2-8. 6 kg (males) and 5.5-6. 8 kg (females). Sexually dimorphic, with males being larger. There have been previous reports of individuals weighing up to 20 kg; either these measurements are incorrect or perhaps from unnaturally heavy captive animals. There is some indication of geographic variation in external measurements across the range of this species, but this needs further substantiation, as sample sizes are not extensive. This animal resembles a small Puma, with a sleek muscular body, long torso, and tail length nearly equivalent to head-body length. Fosa has a relatively short muzzle and short rounded ears. The pelage is fine and relatively dense, generally with uniform, pale, reddish-brown upperparts and dirty cream underparts. In some individuals, particularly males, the venter is colored orange from gland secretions.
Habitat. Fosa is largely forest-dwelling. It is known from near sea level to 2600 m elevation. It occurs in all of the different natural forest types on Madagascar from the wettest (greater than 6000 mm of annual rainfall) to the driest zones (less than 400 mm). This species appears to have higher population densities in the lowland western dry deciduous forests than in the lowland eastern rainforests. Densities decrease with increasing elevation in both sectors. Fosas are also found in the remaining forested islands in the central highlands. They are known on the Andringitra Massif from about 750 m (lower limit of the forest) to 2600 m (above forest line); this latter zone has extreme meteorological conditions with daily temperatures in August ranging from —-11°C to 30°C. Previous reports ofthis species on the off-shore island of Ile Sainte Marie are erroneous: the locality was Sainte Marie de Marovoay, not far from Mahajanga.
Food and Feeding. The Fosa’s large size, considerable strength, carnassial teeth, broad paw pads, highly flexible ankle joints, and semi-retractable claws make it a formidable carnivore, the top predator of Madagascar. In captivity these animals eat 800-1000 g of meat per day. Wild populations have a broad dietary regime, at least in part associated with local prey availability. There appears to be no differences between the sexes in dietary regime. Fosa has been reported to feed on mammals, birds, snakes, lizards, freshwater turtles, and insects. In recent years several analyses of scats have been conducted to determine the diet of this animal, across the different biomes it inhabits. The scats of Fosa are easy to recognize; they form thin 10-14 cm long and 1.5-2. 5 cm wide rolls, with at least one of the ends twisted. Most examples are gray in color; they often contain hair and mammalian bone fragments. At the majority ofsites, mammals are the most important prey. Both by number of individuals and biomass, lemurs are regular elements in the Fosa’s diet; the larger diurnal species of lemurs are at least in part taken from their arboreal sleeping sites. In the dry deciduous forests of Kirindy Centre de Formation Professionnelle Forestiere (CFPF), primates comprise over 80% of the biomass and nearly 60% based on relative frequency of the prey taken. In the dry deciduous forests of Ankarafantsika, larger lemur species (Eulemur spp. and Verreaux’s Sikafa Propithecus verreauxi) are overrepresented in the diet of Fosa as compared to their relative local densities, while notably smaller species (mouse lemurs Microcebus spp. and Lesser Dwarf Lemur Cheirogaleus medius) are underrepresented. At this site there are notable seasonal shifts in the types of lemurs taken by Fosa. Fosas also take a wide variety of non-primate prey and clearly have considerable dietary plasticity. They are known to feed on other Eupleridae ; Fosa was a serious problem in a study of the Spotted Fanaloka, where it followed trap lines and preyed on captured individuals. Bone remains of Narrow-striped Boky have been found in its scats. Fosa can be a voracious chicken predator. During the dry season in western deciduous forests there is a notable increase in the number of chicken remains in scats. Towards a 2000 m summit in the south-east, small tenrecs of the genera Microgale and Oryzorictes (Oryzorictinae), all with adult body mass ofless than 35 g, were found in Fosa scats. Above the tree line in the high mountain zone of Andringitra, birds, rodents, and shrew tenrecs (Microgale) are important components of this animal’s diet; to a lesser extent, their diet includes frogs and crabs. Further, at thissite the average prey body mass is only 40 g, including vertebrates weighing less than 10 g. This contrasts with an average prey body mass of 480 g in the humid forests of the north and 1140 g in the western dry deciduous forests. Some of the prey animals found in the Andringitra scats recovered above tree line were of forest-dwelling animals, indicating that these Fosas have notably large home ranges and do not rely completely on the prey base available above the forest. Fosas are known to take prey nearly their own weight, such as 6 kg lemurs of the genus Propithecus, but assertions that the Fosa is a lemur specialist, with these animals representing more than 50% of its diet, cannot be supported acrossits complete geographic range. Bone fragments of even larger animals, such as cows and bush pigs (Potamochoerus), have been found in Fosa scats and it is presumed that these are scavenged. Fosas have also been cited as feeding on the 1 kg endemic and endangered rodent Votsovotsa Hypogeomys antimena, which is known only from a limited area in central western Madagascar; heavy predation pressure and poor recruitment of young into the breeding population have been cited as reasons for the rodent’s rarity. However, based on the analysis of Fosa scat obtained within this rodent’s range, it represents a small proportion of the Fosa’s diet. Fosa is notably adapted for hunting both on the ground and in trees. It is often seen foraging solitarily, but during the austral spring breeding season has been observed hunting communally; these foraging parties are presumably of male-female pairs. At other times of the year, mothers and their young hunt together. One Fosa will chase lemurs in trees, scaling the trunks and leaping from tree to tree, forcing the lemurs to the ground, where its hunting partners easily catch them. The Fosa’s semi-retractable claws help it grip trees and its long tail, which is not prehensile, acts as a balancing device.
Activity patterns. In the eastern and more mesic portions of the island, Fosa demonstrate highly irregular periods of activity during the day and night and is best considered cathemeral. Two radio-collared individuals showed periods of peak activity soon after dawn, in the mid-afternoon, and late at night. The same general pattern has been found in drier forests in western Madagascar. Solitary individuals do not use the same sleeping sites on a regular basis, but females with young frequent the same den.
Movements, Home range and Social organization. In recent years several studies using radio collars on these animals have been conducted in the dry deciduous forests of western Madagascar. In the Kirindy (CFPF) Forest, north of Morondava, densities have been estimated at one animal per 4 km *; females had home ranges of up to 13 km * and males up to 26 km *. The home ranges of different individuals were not necessarily mutually exclusive, but those of females tended to be separate from one another. Home ranges showed seasonal variation, with notable expansion during the dry season;this is apparently related to food and water availability. The adult sex ratio is 1:1. Animals have been documented to move a straight-line distance of over 7 km in 16 h. On the basis of limited radio-tracking data from the eastern humid forests, home ranges overlapped by approximately 30%. Fosa make few vocalizations, most associated with mating. Olfactory communication seems to be common throughout the year, by scent marking prominent objects such as rocks and trees, or the ground, with a secretion from the anal and chest glands, as well as genitalia. Outside the breeding season, it is rare to find adults together, except for females with their young. Female Fosa show transient masculization: individuals 1-2 years old have an enlarged,spiny clitoris, which is physically supported by a bone-like structure, the os clitoridis, and visually resembles a penis. In these females the masculization does not appear to be associated with a pseudo-scrotum. In adult females the length of the os clitoridis decreases as body length increases. This change is not linked in juvenile (masculinized) and adult (nonmasculinized) females to different levels of hormones (testosterone, androstenedione, dihydrotestosterone). Two different hypotheses have been proposed to explain transient masculization in this species: reduced sexual harassment ofjuvenile females by adult males or reduced aggression from territorial females.
Breeding. Most details of reproduction in wild populations are from the western dry deciduous forests. Copulation occurs between September and December and young are born in December and January. There are conflicting reports of the gestation period in Fosa, ranging from 6-7 weeks to approximately 90 days. Whether certain of these details are applicable to eastern populations will require further field research. Reported observations of copulation in the eastern humid forest were in October. Pairs copulate on horizontal tree branches, generally about 20 cm in diameter and up to 20 m off the ground; there are also reports of intromission on the ground. Mating sites are generally near water sources. Numerous males remain in close vicinity to the receptive female. The female lays belly-down, grasps the substrate with the claws of her forelimbs, with the hind limbs tucked underneath her, and extrudes the genital opening a few centimeters. She gives a series of mewing vocalizations, which seems to stimulate the male to mount her. He mounts from behind, slightly to one side, grasps the female around the waist with his forelimbs, and often licks her neck. There is a copulatory tie, which is difficult to break if the mating session is interrupted. Copulation is repeated several times with a single male and the mating bout can last from one to 14 hours; males often remain close to the females for up to an hour after the sequence has finished. The trees where these annual mating sessions take place are often reused for years, and with remarkable precision as to the date the season commences. As many as eight males can be found around the mating site. With considerable vocalizing, there are antagonistic interactions among them as they compete to have access to the receptive female. Females seem to choose the males they mate with. Their choices are apparently not correlated with morphometric parameters in males, nor do they determine the length of an intromission bout. Over the course of several days a given female will mate with more than one male. In one case, documented by Claire Hawkins, a receptive female remained in the branches of a tree for a week, mating with numerous males. The female was then replaced by another receptive female, who mated with some of the males that had serviced the previous female as well as with newly arriving males. The litter size is generally two, although up to six young have been recorded. Birthing sites include concealed underground dens, rock crevices, or hollows in large tree trunks or termite mounds. Females raise their young alone and have three pairs of nipples—one inguinal, one ventral, and one pectoral. The neonates, weighing less than 100 g at birth, have almost white fur and are blind and toothless. Cub developmentis slow, with the eyes opening about 2-3 weeks after birth. Thereafter they become more active and there is a notable darkening of the fur to a pearl gray color. Litters are of mixed sexes, contrary to some previous assertions. The cubs leave the natal den for the first time around 4-5 months of age and become independent of their mother at about one year. The Fosa gets its permanent teeth at about 1-5 years and the young are sexually mature at 3—4 years of age.
Status and Conservation. CITES Appendix II. Listed in The IUCN Red List as Vulnerable. This species has a broad distribution across much of the forested portions of the island, including a wide variety of vegetational formations and elevational range. On the basis of current information, densities tend to be higher in less-disturbed forests. This species persists in some degraded habitats, which might be related to the considerable distances it can travel on a daily basis. Some Malagasy natives consume this species as bush meat. Fosas are known to prey on domestic fowl and other livestock, but the extent of this predation may be exaggerated. A number of diseases and viruses have been isolated from wild and captive Fosa. Several of these (anthrax, canine distemper, canine parvovirus, feline calicivirus, and Toxoplasma gondi) presumably were transmitted by dogs and cats thatlive in forested habitats and are in contact with Fosa. A combination of factors ranging from habitat destruction to hunting pressure has affected the remaining populations of Fosa acrossits range. Little is known about the genetic structure of wild populations, which is certainly a critical question in the development of long-term conservation programs.
Bibliography. Albignac (1970, 1972, 1973, 1975, 1984), Blancou (1968), Conservation Breeding Specialist Group (SSC/IUCN) (2002), Decary (1950), Dollar (1999a, 2006), Dollar et al. (2006), Garcia & Goodman (2003), Golden (2005, In press), Goodman (2003a), Goodman & Pidgeon (1999), Goodman & Raselimanana (2003), Goodman, Ganzhorn & Rakotondravony (2003), Goodman, Kerridge & Ralisoamalala (2003), Goodman, Langrand & Rasolonandrasana (1997), Goodman, Rasoloarison & Ganzhorn (2004), Grandidier & Petit (1932), Hawkins (1998, 2003), Hawkins & Racey (2005, 2007), Hawkins et al. (2002), Hugh-Jones & de Vos (2002), IUCN (2007), Karpanty & Wright (2006), Kaudern (1915), Kerridge et al. (2003), Kéhncke & Leonhardt (1986), Koéhncke & Schliemann (1977), Laborde (1986), Ljungquist (1930), Louvel (1954), Nicoll & Langrand (1989), Piertney et al. (2000), Powzyk (1997), Rahajanirina (2003), Rasamison (1997), Rasoloarison et al. (1995), Rasolonandrasana (1994), Schreiber et al. (1989), Sommer et al. (2002), Vosseler (1929), Wemmer & Wozencraft (1984), Willis (1895), Wright et al. (1997), Yoderet al. (2003), Youlatos (2003), ZICOMA (1999).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cryptoprocta ferox
| Don E. Wilson & Russell A. Mittermeier 2009 |
Cryptoprocta ferox
| Bennett 1833 |