Tanymastigites lusitanica, Machado, Margarida & Sala, Jordi, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3681.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:28BC003C-9F92-4ADE-983D-EC2DEAB94C15 |
|
DOI |
https://doi.org/10.5281/zenodo.6153997 |
|
persistent identifier |
https://treatment.plazi.org/id/D6072332-855B-D455-FF10-FE8AFDC8B1F7 |
|
treatment provided by |
Plazi |
|
scientific name |
Tanymastigites lusitanica |
| status |
sp. nov. |
Tanymastigites lusitanica sp. nov.
(Figs 1–10)
Tanymastigites sp.—Cancela da Fonseca et al., 2008; Gascón et al., 2012.
Etymology. This species is named after the word Lusitânia, used by one of the greatest Portuguese poets, Luís de Camões, in his masterpiece “Os Lusíadas”, referring to Portugal, where this new taxon was discovered. The gender is feminine.
Type locality. Puddles (for instance, tire tracks) on unpaved clayish roads on the way to and around Horta do Tio Luís temporary pond, Mértola, Alentejo, Portugal (37º45’N, 7º52’W; 159–174 m asl).
Type material. All material preserved in 80º ethanol, although fixed in formaldehyde, except when otherwise stated. All material comes from the district of Beja, Portugal. Holotype: male; HTL (37º45’N, 7º52’W); 17/12/ 2003; TL 26.1 mm, SL 23.0 mm; M. Machado leg.; MB (Accesion number: 11-000931).
Allotype: female; HTL; 09/11/2004; TL 22.3 mm, SL 19.4 mm; M. Machado leg.; MB (Accesion number: 11- 000932).
Paratypes: 1) 3 males; HTL; 30/10/2004; J. Reis & M. Machado leg., M. Machado det.; MB11-000933. 2) 3 males fixed in ethanol; HTL; 26/11/2004; M. Machado leg.; MB11-000934. 3) 3 females; AZ (37º45’N, 7º48’W); 09/11/2004; M. Machado leg.; MB11-000935. 4) 3 males and 3 females; HTL; 09/11/2004; M. Machado leg.; MNCN 20.04/8853. 5) 3 males fixed in ethanol; VF (37º47’N, 7º 48’W); 09/11/2004; M. Machado leg.; MNCN 20.04/8854. 6) 3 males and 3 females; HTL; 26/11/2004; M. Machado leg.; NHMUK 2013.1-6. 7) 3 males fixed in ethanol; VF; 26/11/2004; M. Machado leg.; NHMUK 2013.7-9. 8) 3 males and 3 females; AZ; 09/11/2004; M. Machado leg.; MNHN-IU-2009-3031. 9) 3 males fixed in ethanol; HTL; 26/11/2004; M. Machado leg.; MNHN- IU-2009-3032. 10) 3 males and 3 females; AT (37º46’N, 7º 53’W); 30/10/2004; J. Reis & M. Machado leg., M. Machado det.; ISR CRUS 001TL2013. 11) 3 males fixed in ethanol; HTL; 26/11/2004; M. Machado leg.; ISR. CRUS 001TL2013. 12) 3 males and 3 females; VF; 30/10/2004; J. Reis & M. Machado leg., M. Machado det.; DBUA 1324.01. 13) 3 males fixed in ethanol; VF; 26/11/2004; M. Machado leg.; DBUA 1324.02. 14) 1 male, prepared for SEM; AZ; 16/12/2003; M. Machado leg.; DBUA 1325.01. 15) 2 male and 1 female, prepared for SEM; AT; 27/03/2002; J. Sala & M. Machado leg.; DBUA 1326.01. 16) 1 female, prepared for SEM; AZ; 14/11/ 2003; M. Machado leg.; DBUA 1325.02. 17) Mature cysts, prepared for SEM; HTL; 17/12/2003; M. Machado leg.; DBUA 1327.01. 18) 2 males fixed in ethanol, prepared for SEM; AZ; 10/10/2003; M. Machado leg.; DBUA 1325.03.
Additional material examined. 1) 6 females; HTL; 09/11/2004; M. Machado leg.; MM. 2) 13 males and 9 females; HTL; 26/11/2004; M. Machado leg.; MM. 3) 4 males fixed in ethanol; HTL; 26/11/2004; M. Machado leg.; MM. 4) 4 males and 1 female; AT; 27/03/2002; J. Sala & M. Machado leg.; MM. 5) 5 males; AT; 30/10/2004; J. Reis & M. Machado leg.; M. Machado det.; MM. 6) 1 male fixed in ethanol; AT; 24/04/2002; J. Sala & M. Machado leg.; MM. 7) 4 males; AZ; 16/12/2003; M. Machado leg.; MM. 8) 1 female; AZ; 09/11/2004; M. Machado leg.; MM. 9) 3 males; AZ; 26/11/2004; M. Machado leg.; MM. 10) 4 males fixed in ethanol; AZ; 10/10/ 2003; M. Machado leg.; MM. 11) 1 male fixed in ethanol; AZ; 26/11/2004; M. Machado leg.; MM. 12) 2 males; VF; 18/02/2004; M. Machado leg.; MM. 13) 7 males; VF; 30/10/2004; J. Reis & M. Machado leg.; M. Machado det.; MM. 14) 2 males fixed in ethanol; VF; 09/11/2004; M. Machado leg.; MM. 15) 2 males fixed in ethanol; VF; 26/11/2004; M. Machado leg.; MM. 16) 4 males and 4 females; HTL; 30/10/2004; J. Reis & M. Machado leg.; M. Machado det.; LLAM-GB47. 17) 15 males and 9 females; HTL (puddle #3); 09/11/2004; M. Machado leg.; LLAM-GB46. 18) 7 males and 3 females; HTL (puddle #5); 09/11/2004; M. Machado leg.; LLAM-GB33. 19) 7 males and 3 females; HTL; 26/11/2004; M. Machado leg.; LLAM-GB48. 20) 7 males fixed in ethanol; HTL; 26/11/ 2004; M. Machado leg.; LLAM-GB49. 21) 4 males and 1 female; AT; 27/03/2002; J. Sala & M. Machado leg.; LLAM-GB40. 22) 7 males and 9 females; AT; 30/10/2004; J. Reis & M. Machado leg.; M. Machado det.; LLAM- GB50. 23) 1 male and 1 female; AZ; 20/03/2002; J. Sala & M. Machado leg.; LLAM-GB43. 24) 3 males and 2 females; AZ; 06/10/2002; J. Sala & M. Machado leg.; LLAM-GB41. 25) 4 males; AZ; 14/11/2003; M. Machado leg.; LLAM-GB42. 26) 2 males; AZ; 16/12/2003; M. Machado leg.; LLAM-GB45. 27) 4 males and 2 females fixed in ethanol; AZ; 10/10/2003; M. Machado leg.; LLAM-GB39. 28) 1 male; VF; 18/11/2003; M. Machado leg.; LLAM-GB44. 29) 1 female; VF; 18/02/2004; M. Machado leg.; LLAM-GB51. 30) 3 females; VF; 30/10/2004; J. Reis & M. Machado leg.; M. Machado det.; LLAM-GB52. 31) 1 female; VF; 09/11/2004; M. Machado leg.; LLAM-GB53. 32) 7 males fixed in ethanol; VF; 09/11/2004; M. Machado leg.; LLAM-GB54.
Diagnosis. Male. Antennal appendage with a short, undivided, distally smooth lateral branch; dorsal clypeal process as a non-sclerotized small tubercle, hardly exceeding level of fused basal part of antennal appendages; frontal clypeal process as a well developed dorsoventrally flattened plate; ventral clypeal process as typical of the genus; distal segment of antenna with a short prominent ventrolateral crest at a level very close to the point of maximum curvature; basal part of penis ending into 2 mucronated, divergent “lips”.
Female. 10th and 11th thoracic somites widened, with the 11th somite having one pair of dorsolateral swellings.
Cyst. Spherical, with moderate high crests delimiting irregular polygonal cells, without superficial hairs.
Description. Adult Male. General. Body of living specimens unpigmented, ivory or milky white coloured, sclerotized parts golden brown.
Head with nuchal organ widely elliptic, sometimes almost subrectangular, main axis transversal.
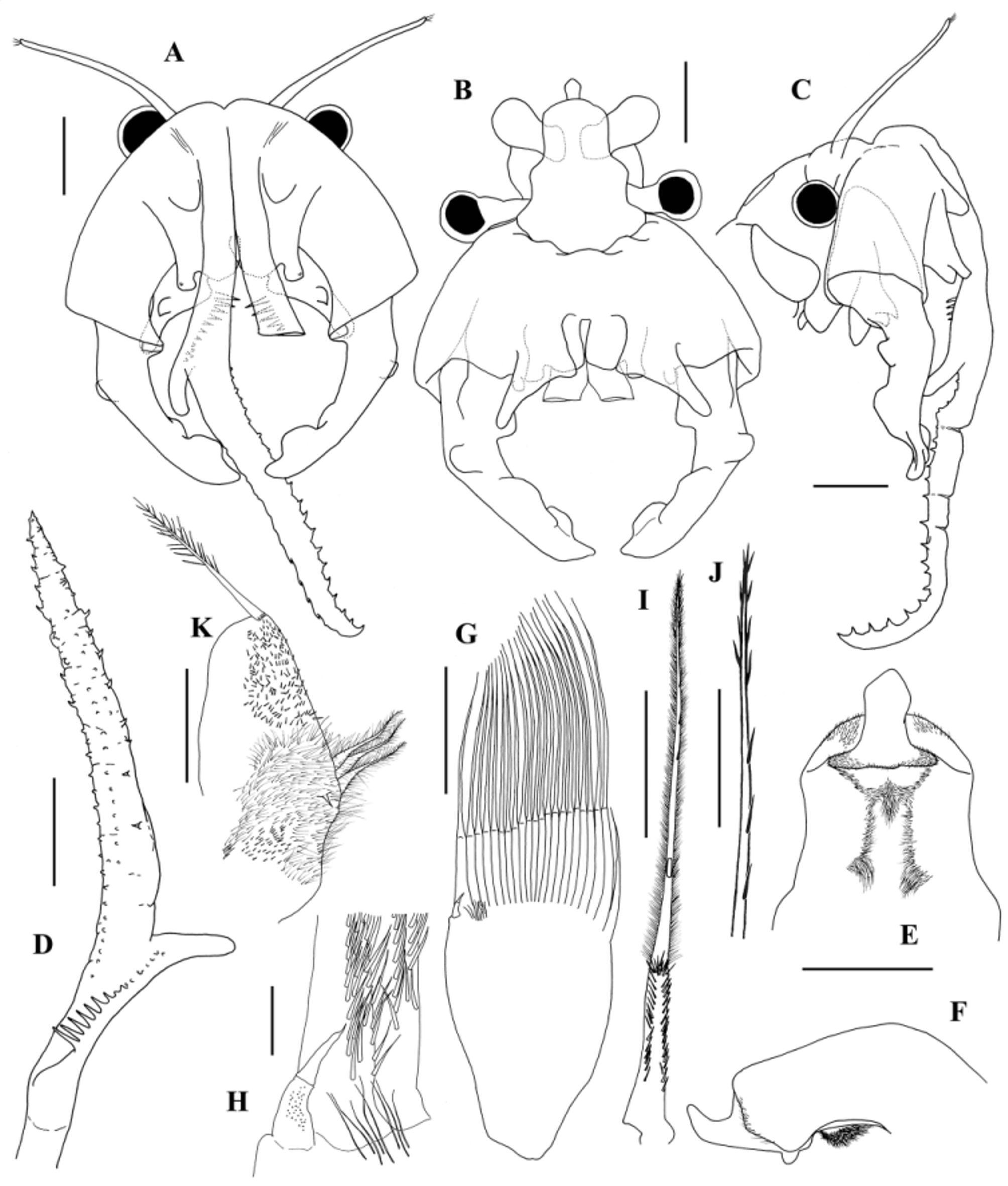
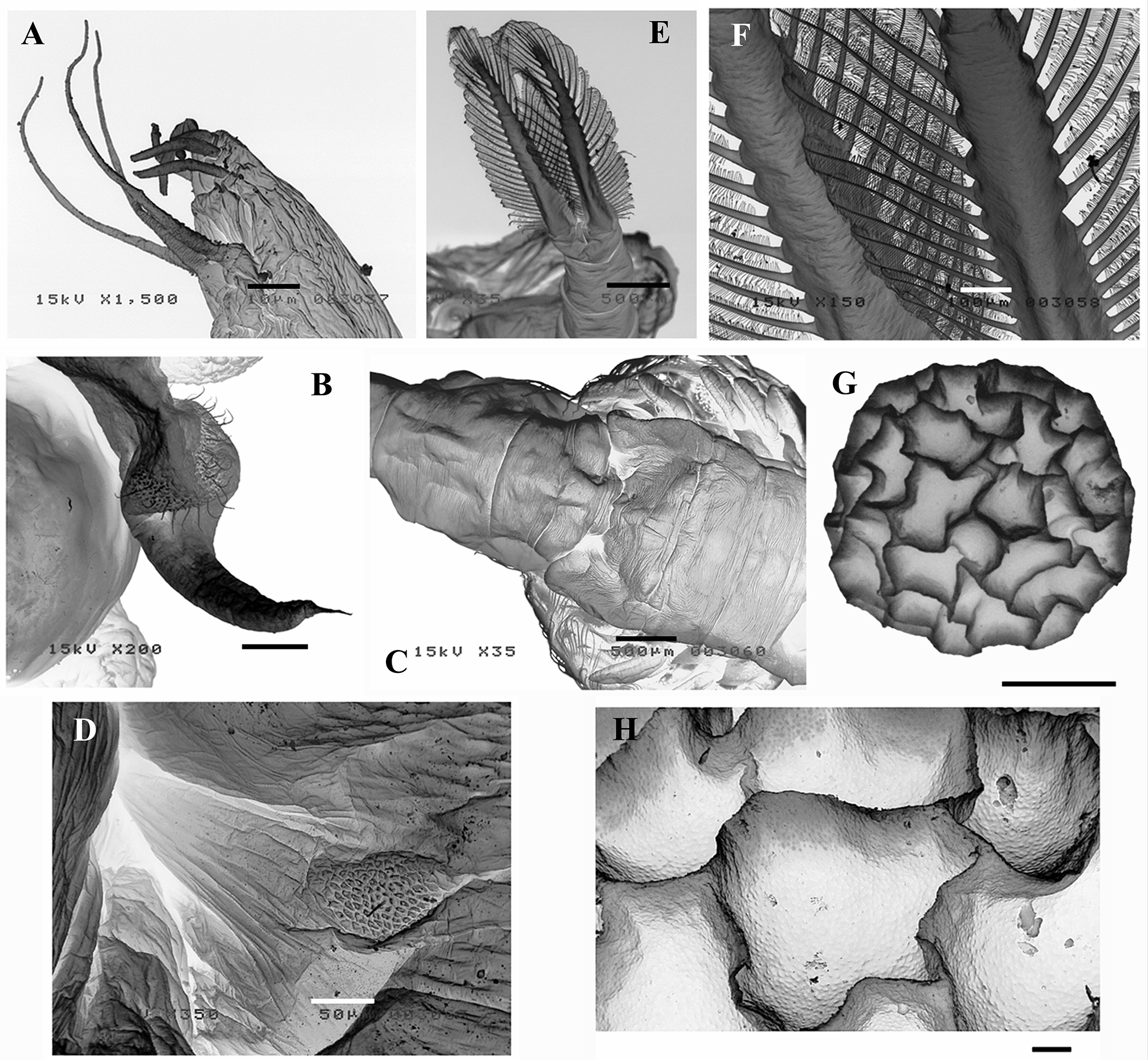
Antennule more than twice as long as the compound eye plus peduncle, longer than the basal segment of antenna ( Figs 2 View FIGURE 2 A, C; 7B), with 3 long subdistal setae and 6 distal aesthetascs (as in Fig. 10 View FIGURE 10 A).
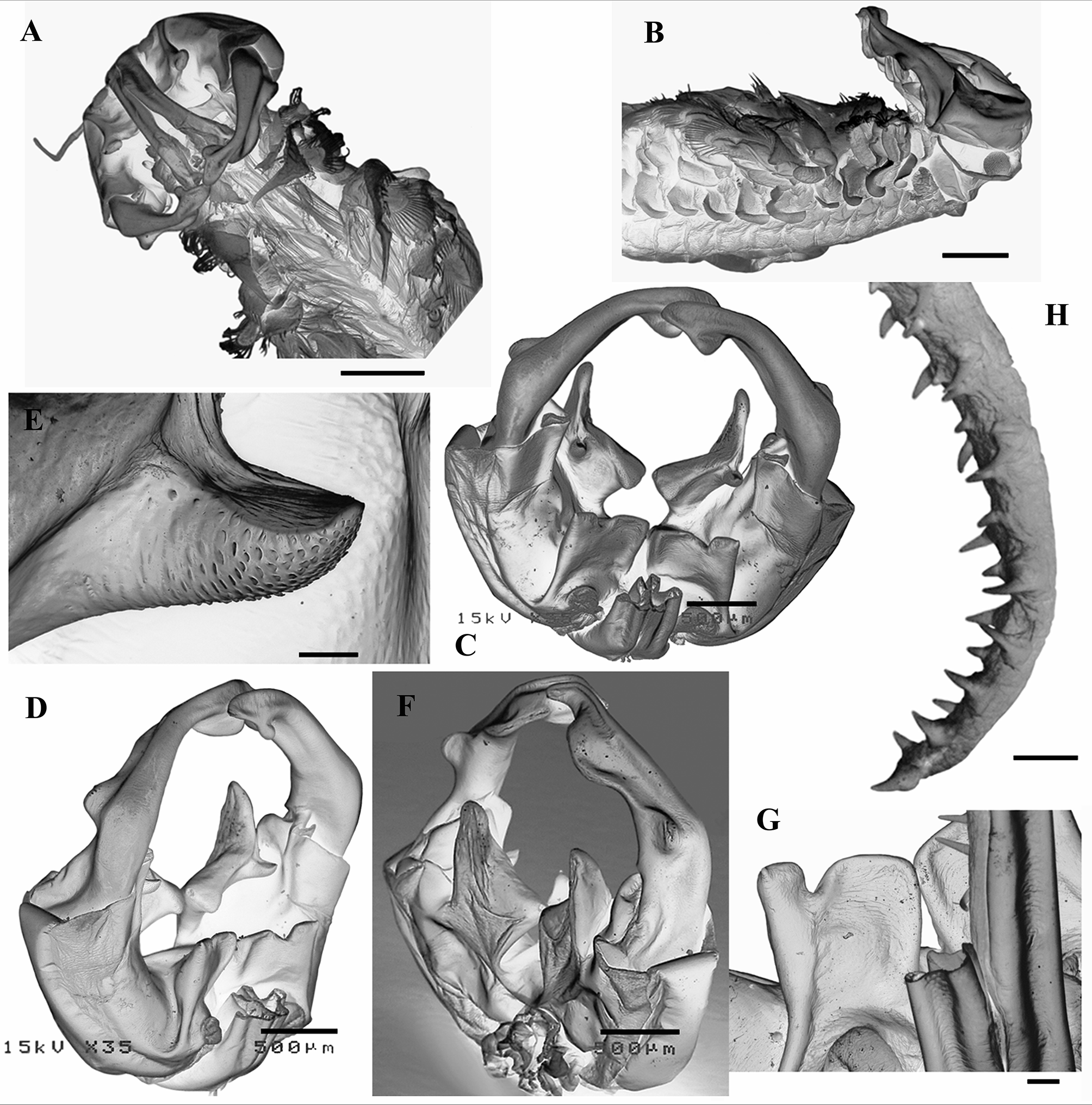
Antennae sclerotized, elliptic (sometimes almost circular) in outline (Figs 1A, B; 2A; 7C), slightly longer than wide, extending up to the posterior limit of 3rd thoracic somite ( Fig. 7 View FIGURE 7 B). Basal segment longer than distal segment of antenna (ratio: 1.1–1.3); proximal 50–60% of basal segment fused, forming the clypeus. Clypeus with 3 pairs of processes (Fig. 1A, B): dorsal (dp), frontal (fp) and ventral (vp). Dorsal processes, located at base of frontal processes as small non-sclerotized rounded tubercles pointing to the front, provided dorsally with a cluster of small setae; they laterally border the incompletely fused proximal part of the antennal appendages, hardly projecting beyond it (Figs 1A; 2A, C; 7C, D, G). Frontal processes partly hidden under the antennal appendages ( Figs 2 View FIGURE 2 A; 7A); each as a subquadrangular plate originating from middle of dorsal extension of clypeus and protruding approximately the same length beyond its frontal end; flattened dorsoventrally, triangular in lateral view and arched dorsally to lateral, mucronated distal angle; medial distal angle a roundish right or somewhat obtuse angle, very close to that of its pair; frontal border with a triangular incision, closer to its lateral side; lateral part of frontal border can extend beyond medial one (Figs 1A; 2A; 7A, C, D, G). Ventral processes arising close to the medial part of frontal border of clypeus, each as a dorsoventrally flattened, subtrapezoidal plate, distal margin longer, medial distal angle right to somewhat acute, widened dorsally into a more or less prominent conical or roundish outgrowth; lateral distal angle prolonged into a digitiform outgrowth extending to or surpassing the medial distal end of the basal segment of antenna; a subconical apophysis on its dorsal side, near its frontal oblique border, approximately midway to distal angles; convex proximal surface of apophysis squamous; frontal flat smooth surface of apophysis approximately transverse, oblique in relation to frontal edge of the plate (Figs 1A, B; 2A, B; 7C, D, E, F). Distal segments of antennae outlining a semicircle, each with the maximum point of curvature at 25– 33% from its proximal end, approximately cylindrical in cross-section diminishing in diameter to the distal end, which is ventrally scooped, spoon-shaped (Figs 1A, B; 2A, B, C; 7C, D, F). Distal segment of antenna with 3 ridges: the most proximal ridge (pr), short and prominent, developing ventrolaterally, at the point of maximum curvature or, more commonly, immediately distal to that point; shaped like a deformed semicircle, flattened (higher) proximally or, less commonly, subtriangular with proximal rounded angle and rounded distal side; concave ventrally and convex dorsally (Figs 1A, B; 2A, B, C; 7A, C, D, F); an intermediate ventral ridge (ir) developing slightly to medial side from the proximal end of distal ventral “spoon” to the base of the most proximal ridge to which it is connected; shaped as an asymmetrical arc of a circle, highest proximally, distal edge somewhat sinuous and flattened (Figs 1B; 2B, C; 7A, B, F); a subdistal ridge (sr) arising ventromedially and developing to dorsomedial side, typically higher distally, subtrapezoidal or subtriangular with roundish free angle and sides or shaped as an asymmetrical arc of a circle, forming a bifurcated extremity with the distal tip of antenna (Figs 1A, B; 2A, B; 7A, C, D). Base of distal segment of antenna with a medial small, unapparent, hyaline lamella (hl), subrectangular, transverse, sometimes prolonged laterally to form a pointed or rounded beak (Figs 1A; 2A; 7C, D). Antennal appendages—also called appendix verticalis in Daday (1910) and Gauthier (1928b), processus serriformis in Brtek (1972) and Thiéry (1986b) or antennal serrated laminar outgrowths in Thiéry & Brtek (1984) —up to twice the length of antennae, arising from the middle of the base of clypeus as an incompletely fused pair of sclerotized cylindroid branches, partially hiding the frontal processes ( Fig. 2 View FIGURE 2 A, C); approximately at the level of half length of these processes, the incomplete fusion of the two antennal appendages ends, and continue squeezed against each other before getting separated like the teeth of a fork closer to the distal edge of frontal processes ( Figs 2 View FIGURE 2 A; 7A, G); from this separation point, each antennal appendage, sclerotized and cylindroid proximally, become progressively wider, flatter and softer, losing the sclerotized appearance before dividing into a very short lateral branch and a long medial branch at 20–33% of the extension of its free portion ( Fig. 2 View FIGURE 2 A, C, D). Antennal appendages, when in resting position, stay wrapped under themselves, enclosed by the antennae ( Fig. 7 View FIGURE 7 A, B); lateral branch digitiform, 14–20% as long as medial branch; medial branch ribbon-like, dorsoventrally flattened, gradually tapering toward a distal, rounded triangular end. Undivided basal part of the free portion of antennal appendage with a longitudinal ventral row of long sclerotized spines standing perpendicular to the appendage surface, rarely bent to medial side; spines getting shorter and softer distally to the base of lateral branch or even until the middle of its length, still forming a single row, or a wider band of short, soft, rounded conical papillae; distal ventromedial edge of undivided basal part of the free portion of antennal appendage and ventromedial edge of medial branch with a row of short, soft, rounded conical papillae becoming small sclerotized spines toward the distal end; ventral side of medial branch ornamented with short, soft, rounded conical or spine-like papillae, distal end normally with one sclerotized spine; ventrolateral edge of medial branch with more or less sclerotized spines, normally getting shorter toward its distal end; marginal spines of distal part of medial branch (ventromedial, distal and ventrolateral) approximately of same size ( Figs 2 View FIGURE 2 A, C, D; 7H).
Labrum very similar to that of Branchipus cortesi Alonso & Jaume ; distal lamella widest subdistally, ending in a rounded acute angle ( Figs 2 View FIGURE 2 E, F; 8A).
Mandibles as in Fig. 9 View FIGURE 9 , similar to those described for the genus ( Mura, 1996).
First maxillae, each with 22–24 setae and a posterior ventral spine ( Fig. 2 View FIGURE 2 G); basal part of setae half length the distal part, or a little less; posterior side of the basal part of setae completely covered with spines arranged in two rows; transitional area between basal and distal part of setae also completely covered by spines ( Fig. 2 View FIGURE 2 I); anterior side of distal part of setae ornamented with two rows of fine setulae ( Fig. 2 View FIGURE 2 I), incorporating in the distal half more spaced spines ( Fig. 2 View FIGURE 2 J); posterior ventral spine small (around 0.1 times the shortest setae), devoid of setulae, divided into two subequal parts, and ending in a fine point; a field of fine setulae appear near the base of the posterior ventral spine ( Fig. 2 View FIGURE 2 G, H).
Second maxillae ventrally directed, swollen, truncated distally; proximal part widely covered with fine setulae, with a small spine and 2–3 anterior setulose setae; distal part covered with scattered small setulae, and a ventral distal long plumose seta with basal crown of pectinate scales ( Fig. 2 View FIGURE 2 K).
Thorax fusiform, wider at 6th or 7th somite level. Eleventh thoracic somite with lateral lobes at the posterior 20– 25% of its length. Dorsally, each thoracic somite carries one pair of warty outgrowths with a central sensilla, similarly to other anostracan species ( Linder, 1941).
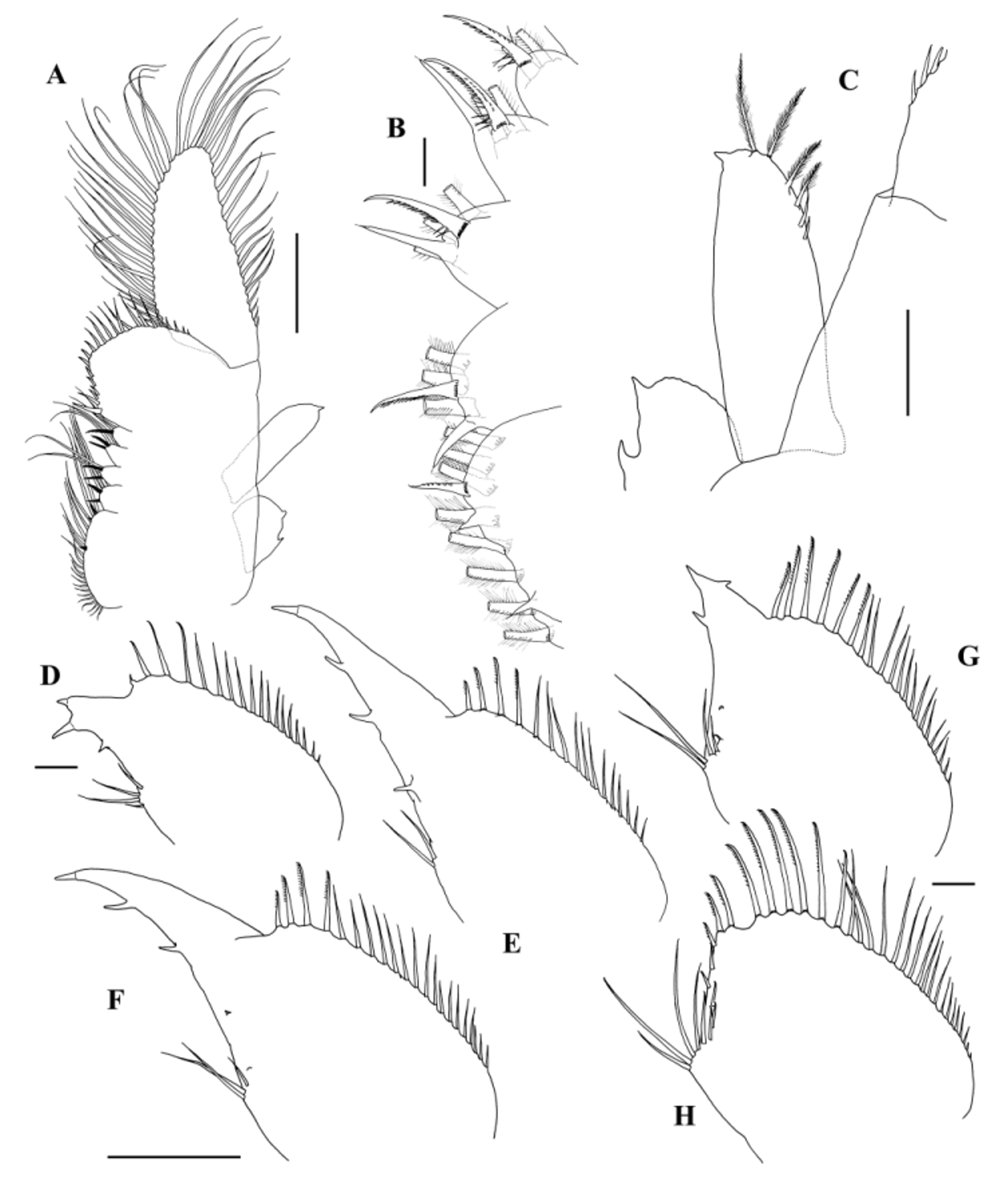
Thoracopods are homonomous, though T4–T7—less commonly T8—are the biggest ones ( Fig. 3 View FIGURE 3 A). Praepipodite undivided, ear-shaped in the first ten thoracopods, about twice as long as high, with 2 spaced denticles on lateral edge and denticulate distal edge (Fig. 1C; 3A, C, F); praepipodite of T11 as a basally truncated ellipsis, oblique, directed to the distal part of thoracopod, about 1.5 times as long as high, edge almost smooth except for 2 denticles on lateral margin ( Fig. 4 View FIGURE 4 A).
Epipodite tending to be longer in relation to width (from about 2:1 to about 3:1) from anterior to posterior thoracic appendages, digitiform in the first ten thoracopods, margin smooth ( Fig. 3 View FIGURE 3 A, C, F); epipodite of T11 of similar shape but usually narrowing towards distal tip, with a denticle on it ( Figs 3 View FIGURE 3 A; 4A, C); the distal part of its medial edge (much more rarely of lateral edge) may present a few naked or plumose setae ( Fig. 4 View FIGURE 4 C): 36.4% of the examined males (n=151) have one or both epipodites of T11 with those setae; this percentage differs from a population to another, ranging from 12.5% (n=32) in VF, to 57.1% (n = 28) in AZ.
Exopodite like an asymmetrical truncate orange segment, wider near the base, straighter side lateral, tending to be longer from anterior to posterior thoracic limbs (length of exopodite in relation to length of endopodite, from about 0.8 times at T1 to about 1.8 times at T11; length of exopodite in relation to length of the remaining of thoracopod, from about 0.8 times at T1 to approximately equal at T11; length of exopodite in relation to length of the whole thoracopod, from about 0.4 times at T1 to 0.9 times at T11) ( Figs 3 View FIGURE 3 A, C, F; 4A); provided with a few (5–7) proximal spine-like setae on lateral margin, gradually longer distally; rest of exopodite with marginal plumose setae.
Endopodite of T1 semi-oval ( Fig. 3 View FIGURE 3 A, C); endopodite of T2 elongated semi-oval with a small distomedial subtriangular projection ( Figs 3 View FIGURE 3 A; 4D); endopodite of T3–T4 elongated semi-oval with a pronounced narrow distomedial subtriangular projection ( Figs 3 View FIGURE 3 A; 4E, F; 7A); endopodite of T5–T10 expanded semi-oval, medial edge straightened with an angulation closer to the 6th endite, apex provided with a notch ( Figs 3 View FIGURE 3 A, F; 4H); endopodite of T5 sometimes also with a small narrow distomedial subtriangular projection ( Fig. 4 View FIGURE 4 G), intermediate shapes rare but possible; endopodite of T11 as that of T5–T10, but apex without a notch ( Fig. 4 View FIGURE 4 A); endopodite, excluding the subtriangular projection on T2–T4 or T5, about half length of the whole thoracopod; endopodite of T1 provided with spine-like setae on medial or distomedial margin and plumose setae on lower margin; endopodites of T2–T4 (also, that of T5 when it presents a distomedial subtriangular projection) with spine-like setae on medial margin and on distal part of lower margin, plumose setae on proximal part of lower margin and some short, naked spines (3–4) on the subtriangular projection; endopodites of T5–T10 with spine-like setae on distomedial margin and on distal part of lower margin and plumose setae on proximal part of lower margin; endopodite of T11 with spine-like setae on distomedial margin and plumose setae on lower margin.
Endite 1+2 elongated with roundish edge bordered by a comb of long filtering setae lacking basal crown of pectinate scales except at T11, and with the typical anostracan number and location of anterior setae ( Linder, 1941). The distal anterior seta, strong, pectinate, with basal crown of pectinate scales. The intermediate anterior seta at T1–10, very close to the distal one, is very short, bubble-like and naked; at T11, it is not so close to the distal one, almost half length of it, conical, ending in a stiletto. The most proximal anterior seta at T1–T10, approximately twice as long as the most distal anterior seta, fragile and provided with sparse, short and very fine setulae at both sides; at T11 it is similar in shape to the intermediate seta, about half length of the most distal anterior seta ( Figs 3 View FIGURE 3 D, G; 4B).
Third endite normally 25–33% as long as endite 1+2, with roundish edge bordered by a comb of long filtering setae at T1–T10, with the typical anostracan number and location of anterior setae ( Linder, 1941). The more distal anterior seta similar to the more distal anterior seta of endite 1+2, about twice its length, and the more proximal one of the same type as the intermediate seta of T11 endite 1+ 2 ( Fig. 3 View FIGURE 3 D, G). T11 with 3rd endite globular just a little longer than the 4th–6th endites; filtering comb reduced to 2–4 long setae; anterior setae similar to the corresponding setae at the other thoracopods, the more distal one located near the middle of the endite margin and about 1.5 times as long as the more distal anterior seta of endite 1+ 2; the more proximal one close to endite 1+ 2, a little shorter or as long as the more distal one ( Fig. 4 View FIGURE 4 B).
Fourth endite conical (rounded conical at T1; rounded conical to globular at T11) with 2 spine-like anterior setae and typically 3 (2–3) long plumose posterior setae located distally at the first ten thoracic limbs, and 2–3 located centrally at T11 ( Figs 3 View FIGURE 3 E, H; 4B).
Fifth endite conical or rounded conical, with 2 spine-like anterior setae and typically 2 (1–3) long plumose posterior setae located distally at the first ten thoracic limbs, and normally in the middle of the endite at T11 ( Figs 3 View FIGURE 3 E, H; 4B).
Sixth endite typically conical (sometimes rounded conical) at T1–T10, and rounded conical (sometimes conical or globular) at T11, with 1 spine-like anterior setae and typically 2 (1–2) long plumose posterior setae located distally (sometimes closer to a central position) at the first 10 thoracic limbs and 1–2 posterior setae located centrally at T11 ( Figs 3 View FIGURE 3 E, H; 4B).
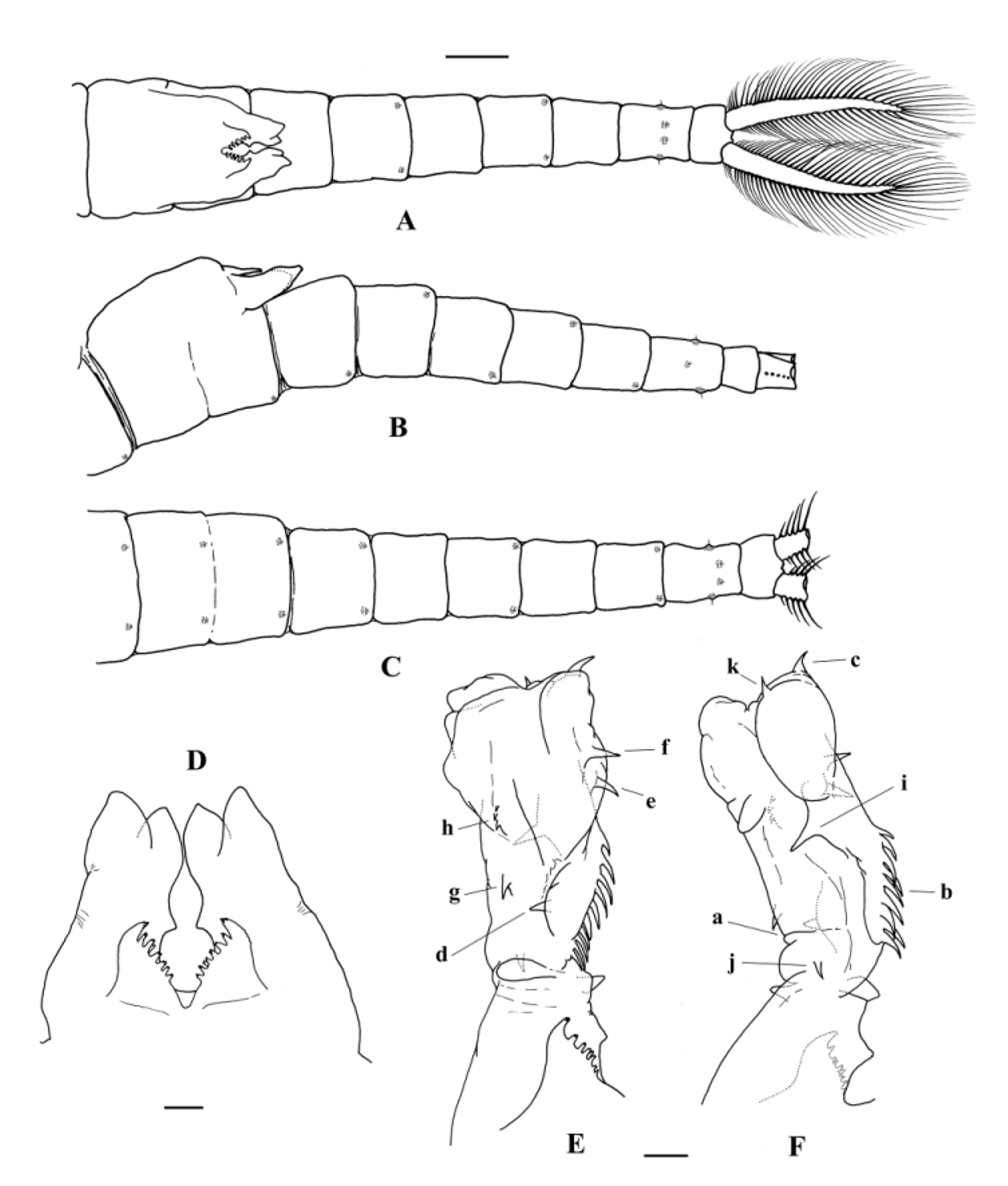
Genital somites. First genital somite usually longer than 11th thoracic somite and wider than the 2nd genital somite, both with posterodorsolateral pair of warty outgrowths with a central sensilla ( Fig. 5 View FIGURE 5 C). Penes with a pair of symmetrical, ventral basal, dorsoventrally flattened, triangular sclerotized processes typical of the genus ( Brendonck, 1995), with lateral margin regularly curved or, when forming an angle, more obtuse than the one described for T. brteki ( Thiéry, 1986b) ( Figs 5 View FIGURE 5 A, D; 8B, C). Basal part of each penis cylindroid, extending posteriorly hardly up to the end of 1st postgenital somite, about twice as long as the ventral basal triangular process, and with the medial spur typical of the genus at a level just proximal to the distal end of the triangular process; distal end with 2 mucronated “lips”, the lateral “lip” longer than the medial, mucrons divergent ( Figs 5 View FIGURE 5 A, D; 8B). Apical eversible part of penis ( Figs 5 View FIGURE 5 E, F; 8D, E, F) about twice as long as its basal part, extending as far as the end of the 3th postgenital somite; cylindroid with the distal part wider, lobed, with a transversal proximal constriction (a) at 20–25% of its length and a conspicuous ridge (carina: b) along most of the non-distal lobed part of the medial side, disrupting the transversal constriction; conspicuous longitudinal medial carina provided with a normally single row of big spines (7–14; n=48) typically directed to the base of the penis, the more distal the shortest; also medially, one medium or long apical spine (c) in the wider distal part of everted penis; ventral side with one big spine approximately at level of the transversal constriction (d), normally with one (0–2) subdistal spine of variable size (e) and with one long, more distal spine closer to the medial margin (f), proximal in relation to spine c; ventrolateral side typically with one medium or long spine distal to transversal constriction (g) and one or more small spines (usually 2–5) proximal to the wider lobed part of protruded penis, in discrete groups or longitudinal row(s) (h); dorsal side with one big medium-distal spine (i) and typically with another one, proximal to transversal constriction, directed to the penis base (j); also dorsally, there is normally 1 (0–3) small or medium sized apical spine (k); the pointing direction of most of the spines depends on the degree of tumescence of the everted part of penis; each everted portion of penis is rarely perfectly symmetrical to its pair.
Postgenital somites gradually decreasing in width to the posterior end; each of the postgenital somites carries one to several pairs of warty outgrowths with a central sensilla; first, 3rd and 5th postgenital somites present a posterodorsolateral pair, 2nd and 4th postgenital somites with a posteroventrolateral pair; 6th postgenital somite, longer than the 5th, with 3 pairs: one lateral and one ventrolateral normally at 60% of the length of the somite, and one dorsal, slightly posterior than the other 2 pairs ( Fig. 5 View FIGURE 5 A, B, C). Telson wider posteriorly, shorter than the last postgenital somite ( Fig. 5 View FIGURE 5 A, B, C). Cercopods ensiform, often slightly convergent, normally as long as telson plus the last 2 postgenital somites (relative length depends on the degree and kind of contraction of the body after fixation), uniformly provided with plumose setae all around the margin, the distal ones being the longest ( Fig. 5 View FIGURE 5 A; or as in Fig. 10 View FIGURE 10 E).
Adult Female. General. Alive individuals with unpigmented body, same colour as males, brood pouch rusty brown, oviduct turquoise blue.
Head rounded, nuchal organ elliptic, as in male.
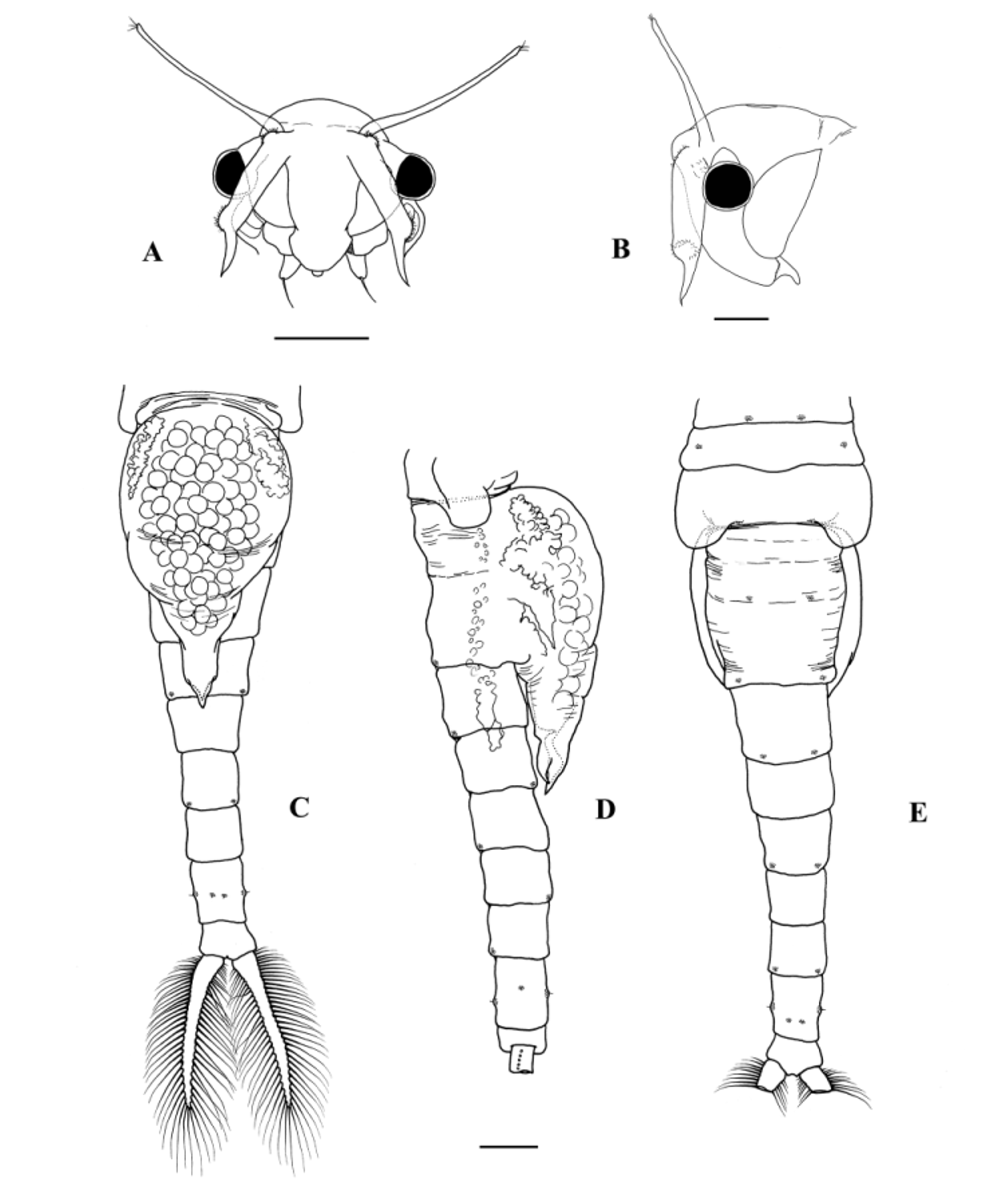
Antennule s filiform, a little longer than the antennae, bearing 3 long subterminal setae and 6 terminal aesthetascs ( Fig. 10 View FIGURE 10 A).
Antennae about twice as long as compound eye plus peduncle, cylindrical ending ventromedially into an acute and curved apex tapering to a sharp point; cylindrical part of antennae proximally and distally slightly swollen with dorsolateral clusters of short sensorial setae; acute apex about 25% of the total length of antennae ( Figs 6 View FIGURE 6 A, B; 10B).
Labrum, mandibles and maxillae as in male.
Thorax. Thoracic somites of similar length, except the 10th and 11th, which are about 1.2 and 1.5 times longer than the 9th, respectively; hardly increasing in width until the 9th, 10th and 11th somites typically 1.2 times wider than the 9th somite. Eleventh segment with a posterior dorsolateral swelling in each side, posteriorly rounded, coinciding with smaller anterior dorsolateral swellings in the first genital somite, both swellings enclosing the amplexial groove in each side; medial side of each posterior dorsolateral swelling of eleventh somite with a warty outgrowth with a central sensilla, just at the entrance of the amplexial groove ( Figs 6 View FIGURE 6 D, E; 10C, D). Each of the other thoracic somites with one pair of dorsal warty outgrowths with a central sensilla, located as in males.
Thoracopods similar to those described for the male, except for the shorter size of the endopodites (endopodites about 40% of the length of the whole thoracopod in T1–T8, and around one third in T9–T11), and the absence of the distomedial subtriangular projection of the endopodites in T2–T4 (and T5 when present) ( Fig. 3 View FIGURE 3 B). The females also can exhibit setae in the distal part of the medial edge of the epipodites in T11, but less commonly than males (16.9% of the examined females (n=71)).
Genital somites partially fused, barrel-like, with some dorsal remains of the segmentation; each genital somite usually longer than the postgenital somites, although first genital somite can sometimes be as long as first postgenital somite; second genital somite typically 1.1 times longer than the first genital somite ( Figs 6 View FIGURE 6 D, E; 10C); each genital somite with a pair of dorsal warty outgrowths. Brood pouch arising from both genital somites, elongated, extending as far as half the length of the 3rd postgenital somite, fusiform in lateral view, tapering to a point; distal part of the brood pouch more or less parallel-sided in ventral view; pore opening subdistally, enclosed between two acute lips, with ventral lip longer than dorsal one ( Fig. 6 View FIGURE 6 C, D).
Postgenital somites gradually decreasing in width to the posterior end; 1st postgenital somite normally a little longer than the 6th postgenital somite, the other postgenital somites about 0.8 times shorter; warty outgrowths with a central sensilla present in all the segments, in a similar arrangement as in the male: one posterodorsolateral pair in 1st, 3rd and 5th postgenital somites; one posteroventrolateral pair in 2nd and 4th postgenital somites; three pairs in 6th postgenital somite—1 lateral, 1 posteroventral, and one posterodorsal ( Fig. 6 View FIGURE 6 C, D, E). Telson and cercopods as in male ( Figs 6 View FIGURE 6 C; 10E, F).
Cyst. Average cyst diameter 348 µm (range=293–373 µm, n=516), spherical, with high ridges defining polygonal depressions of several shapes and sizes; ridges usually higher at the confluent points. Surface of depressions and ridges covered with small pits ( Fig. 10 View FIGURE 10 G, H). Colour of mature cysts is pale brown. No significant differences in cyst size between small (mean TL= 15.4 mm, n=5) and large (mean TL=18.0 mm, n=5) females were found (F1,8 = 0.193, p = 0.672).
Size. Medium-sized fairy shrimps. Males: TL, 13.6–26.1 mm (n=115); SL, 12.2–23.0 (n=115). Females: TL, 11.0– 26.7 mm (n=72); SL, 9.6–22.9 mm, (n=72).
Differential diagnosis. Males of Tanymastigites lusitanica sp. nov. can be easily separated from the rest of the species of the genus by the morphology of antennae and penes. T. perrieri (Daday) is the closest species to T. lusitanica sp. nov. The most striking differences are the absence of a proximal ventrolateral short ridge close to the point of maximum curvature of the antennal distal segment, in T. perrieri , and the shape of the distal lateral “lip” of basal part of penis, which, in T. perrieri , is a big thorn-like outgrowth pointing medially, and in T. lusitanica sp. nov. has a small conical mucron pointing laterally. Moreover, the lateral branch of the antennal appendage is much longer in T. perrieri than in the new species (about half the length of the medial branch in the former, and not longer than 0.2 times the medial branch in T. lusitanica sp. nov.), and its distal tip being spiny in T. perrieri and smooth in the new species. Also, the dorsomedial hyaline lamella in the base of the distal segment of antenna is more developed in T. perrieri , being longer than wide, with a distal pointed tip.
Males of Tanymastigites lusitanica sp. nov. can be immediately distinguished from T. brteki Thiéry , T. cyrenaica Brtek and T. mzabica (Gauthier) by the shape of the antennal appendage, which in these 3 species has a bifurcated lateral branch. T. lusitanica sp. nov. can also be separated from T. brteki by the clypeal processes, the dorsal ones being sclerotized and much more prominent, in relation to the basal part of antennal appendages, in T. brteki ; in T. lusitanica sp. nov., the frontal processes are much more developed, and the ventral ones more delicate than those of T. brteki . Also, the spines forming a ventral longitudinal row in the undivided basal part of antennal appendage are much smaller in T. brteki . The distal segment of the antenna of the new species closely resembles that of T. brteki , although its distal tip is less clearly bifurcated and the medial hyaline lamella on its base is more developed in T. brteki than in the new species. Also, the distal mucron in the “lips” of basal part of penis are absent or unapparent in T. brteki . The second maxillae are also useful to separate T. brteki from T. lusitanica sp. nov. In T. brteki (both sexes), the second maxillae are much more elongated than in T. lusitanica sp. nov.
The lack of information on the morphology of females of some Tanymastigites species does not allow us to establish clear diagnostic features for the differentiation of all species. Thiéry (1986b) mentioned the absence of lateral swellings in the posterior thoracic somites in T. perrieri , contrasting with their presence in T. brteki . In T. lusitanica sp. nov., those swellings develop posterior and dorsolaterally, as a result of the widening of the 10th and 11th thoracic somites in relation to the anterior ones, similarly to T. brteki . However, in T. brteki , the 11th thoracic somite presents laterally a distinctive transversal constriction, which produces two characteristic swellings, in dorsal view: the proximal spherical, and the distal as a pointed wing (as a wide, posteriorly roundish wing, in lateral view), bordering the amplexial groove ( Thiéry, 1986b); this is more clearly observed in large females. In T. lusitanica sp. nov., only the distal swelling is present, with the margin evenly rounded, but similar in length to that of T. brteki . In T. perrieri , posterior dorsolateral swellings also appear in the 11th thoracic somite bordering the amplexial groove, but they are weakly developed. In live females, the lateral sides of the brood pouch have a pale green blotch in T. brteki , whereas in T. lusitanica sp. nov., this blotch is turquoise blue, with the color being preserved during some time in formalin-fixed specimens.
There is no information about the cysts of T. mzabica . Those of T. cyrenaica are spherical, hairy and smooth ( Thiéry, 1996). Cysts of T. perrieri , T. brteki and T. lusitanica sp. nov., spherical as well, are ornamented with ridges delimiting polygonal depressions. The cysts of T. lusitanica sp. nov. are smaller (mean diameter= 0.35 mm, range= 0.29–0.37 mm, n=516) than those of T. perrieri (mean diameter= 0.40 mm, range= 0.37–0.43 mm; Thiéry & Brtek, 1984) and T. brteki (mean diameter= 0.37 mm, range= 0.35–0.40 mm; Thiéry, 1986b). The cysts of T. brteki present more numerous, smaller regular polygons and shallower crests than those of T. perrieri and T. lusitanica sp. nov. Moreover, crests in T. brteki cysts are hairy ( Mura & Thiéry, 1986; Thiéry, 1986b). Concerning external morphology, cysts of T. lusitanica sp. nov. can not be clearly distinguished from those of T. perrieri , not even on the base of difference in size, as there is an overlap between their size ranges.
Distribution and habitat. To date, the species is known from nine puddles or set of puddles on unpaved roads located in a small central area (1172 km 2) of South Alentejo, Portugal, ranging from 37º45’N to 37º57’N and from 7º48 W to 8º24’W, at an altitude of 90 to 185 m asl ( Fig. 11). Apart from the localities used for the description of the species (Horta do Tio Luís, Atafona, Azinhal, and Vale Fresco), it is also known from three other sites close to Beja (Monte da Lagoa, Lagoa Velha and Corte de Azinha), and two more sites near Alvalade (Amoreira-Peneireiro and Defeza). T. lusitanica sp. nov. has always been found in small (mean area= 10.5 m 2; range=1.7–87.0 m2; n=25) and very shallow (mean depth= 16 cm; range= 5–31 cm; n=25) puddles or rainpools on unpaved clayish or sandy roads. Typically, the water is very turbid (mean turbidity=1,410 NTU; range=41.2–16,920 NTU; n=21), low mineralized (mean water conductivity (EC25)=94.6 µS·cm -1; range=16.1–594 µS·cm -1; n=21) and poorly colonized by aquatic vegetation (plant cover <25%). During daytime, between the end of October until the end of March, we found a mean water temperature of 18ºC, ranging from 12.5 to 30.1ºC (n=21), a mean pH of 7.0 (range=6.3–8.9; n=21) and a mean dissolved oxygen saturation of 77.5% (range=32.6–152.9%; n=21).
Most frequently, Tanymastigites lusitanica sp. nov. is the only large branchiopod species found inhabiting those puddles. It was found with Triops baeticus Korn in only one site. Occasionally, some sections of unpaved roads located around temporary ponds were flooded by the rising water level of the ponds thereof. In this situation, some specimens of the fauna of large branchiopods from the pond reached those areas and stayed there after the lowering of the water level, with the puddles getting isolated again from the pond water body. In such cases, we found T. lusitanica sp. nov. sharing its habitat with Branchipus cortesi Alonso & Jaume , Chirocephalus diaphanus Prevost and Triops baeticus . Also, on those occasions, the vegetation cover could increase and water could be much more transparent. Nevertheless, after those not so rare floodings, we never found T. lusitanica sp. nov. in the neighbouring temporary pond.
T. lusitanica sp. nov.
Life history. Not much is currently known about the life history of Tanymastigites lusitanica sp. nov. At the type locality (HTL), by the end of October 2004, we found adult males and females of 17–20 mm TL (measured after fixation) 12 days after the first rainy day. Ten days later, in the same puddle, we found two generations of adults: the older individuals with 20–21 mm TL and the younger ones with 15–18 mm TL; in the same day at another HTL puddle we found adult males and females with 13–14 mm TL. This suggests that Tanymastigites lusitanica sp. nov. can mature in less than 12 days, its life cycle can be as long as 3 weeks, and that several generations can develop before the puddle dries out. The swimming behaviour of this species seems to be slightly different compared with the other anostracan species present in the area, as they are very calm swimmers, and they do not immediately react to the presence or appearance of disturbing elements.
IUCN Red List status. Although this species appears in very ephemeral and marginal habitats (and hence, some populations could have been overlooked), an intensive survey was carried out in the area allowing to identify only nine populations. Thus, Tanymastigites lusitanica sp. nov. meets the IUCN Red List criteria ( IUCN, 2001) for considering the species as Vulnerable, with an area of occupancy less than 2000 km 2, less than 10 locations, and extreme fluctuations in the area of occupancy (VU B2ac(ii)). The fact that T. lusitanica sp. nov. has been found exclusively in puddles located in unpaved roads makes them very susceptible to disappear if the roads are paved. However, we think that some degree of disturbance (due to trampling by livestock or people, or very low frequency of vehicles) in these unpaved roads are necessary for the survival of the populations, as this species is absent in puddles where there is no circulation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |