Pseudopaludicola florencei, Andrade & Haga & Lyra & Leite & Kwet & Haddad & Toledo & Giaretta, 2018
|
publication ID |
https://doi.org/10.11646/zootaxa.4433.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:5A9710FB-C2A6-4A8D-8B1E-D074142C1FFB |
|
DOI |
https://doi.org/10.5281/zenodo.6489592 |
|
persistent identifier |
https://treatment.plazi.org/id/DA1DCB73-7A40-FFF5-D5F9-FF26C71DFE34 |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudopaludicola florencei |
| status |
sp. nov. |
Pseudopaludicola florencei View in CoL sp. nov.
( Figures 2–3 View FIGURE 2 View FIGURE 3 , Tables 1–2)
Pseudopaludicola View in CoL sp. (Andaraí/BA): Duarte et al. 2010; Andrade et al. 2016
Pseudopaludicola View in CoL sp. 1 (Andaraí/BA): Veiga-Menoncello et al. 2014
Holotype. Adult male ( ZUEC 23521 View Materials ; Figures 2–3 View FIGURE 2 View FIGURE 3 , call voucher) collected by F.S. Andrade and A.A. Giaretta in Andaraí ( 12°48’22.28” S, 41°19’59.47” W, 416 m a.s.l.), Chapada Diamantina, state of Bahia, Brazil on 26 November 2016. GoogleMaps
Paratypes. Thirteen adult males: ZUEC 23512–3 , 23515 , 23518–20 , 23522 , 23524–5 , 23527–30 ; and five adult females: ZUEC 23514 View Materials , 23516–7 View Materials , 23523 View Materials , and 23526 , all collected with the holotype. GoogleMaps Seven specimens: UFMG 4310–6 View Materials , collected at Área de Proteção Ambiental Marimbus-Iraquara, Andaraí, state of Bahia, Brazil ( 12°45’46” S, 41°17’48’’ W, 326 m a.s.l.; ca. 6 km from the type locality) on 26 January 2010 by F. S. F. Leite. GoogleMaps
Additional specimens: Brazil: state of Bahia: municipality of Mutuípe: CFBH 29652 (adult male); state of Minas Gerais: municipality of Nanuque: CFBH 33235 (adult female).
Diagnosis. Pseudopaludicola florencei sp. nov. is assigned to the genus Pseudopaludicola by having a hypertrophied antebrachial tubercle (see Lynch 1989, Lobo 1995). The new species is characterized by the following combination of characters: (1) small size (SVL 12.8–14.8 mm in adult males); (2) upper eyelids smooth, with no palpebral tubercles; (3) heel smooth, with no enlarged conical tubercle; (4) relatively short hindlimbs (tibiotarsal articulation just reaching the posterior margin of eye); (5) 11 pairs of chromosomes (2n=22; see Duarte et al. 2010); and (6) advertisement call composed of regular series of three-pulsed notes, emitted at high rate.
Comparison with other species. Pseudopaludicola florencei sp. nov. is promptly diagnosed from the P. pusilla species group (see Lynch 1989), which includes P. boliviana Parker , P. ceratophyes Rivero and Serna , P. llanera Lynch , P. pusilla (Ruthven) , and P. motorzinho Pansonato, Veiga-Menoncello, Mudrek, Jansen , Recco- Pimentel, Martins, and Strüssmann by its terminal phalanges knobbed; whereas the abovementioned species have T-shaped terminal phalanges or expanded toe tips (disks or pads). The tips of the phalanges of the new species are similar in shape to those of P. falcipes ( Figure 2B View FIGURE 2 in Cardozo & Suárez 2012). The new species is also distinguished from P. ceratophyes by having upper eyelids smooth; P. ceratophyes has upper eyelids with an enlarged palpebral tubercle ( Lynch 1989). Pseudopaludicola florencei sp. nov. differs from P. boliviana and P. motorzinho also by the heel smooth, with no enlarged, conical tubercle on the heel ( Pansonato et al. 2016).
Pseudopaludicola florencei sp. nov. is distinguished from P. saltica (Cope) , P. murundu Toledo, Siqueira, Duarte, Veiga-Menoncello, Recco-Pimentel , and Haddad, and P. jaredi Andrade, Magalhães , Nunes-de-Almeida, Veiga-Menoncello, Santana, Garda, Loebmann, Recco-Pimentel, Giaretta, and Toledo ( P. saltica species group) by having relatively short hindlimbs; i.e., the tibiotarsal articulation reaches the posterior margins of eye in the new species, whereas it reaches the tip of the snout in the P. saltica species group.
The chromosome number 2n = 22 distinguishes Pseudopaludicola florencei sp. nov. (as Pseudopaludicola sp. in Duarte et al. 2010) from P. mystacalis (Cope) (2n = 16); P. canga Giaretta and Kokubum , P. facureae Andrade and Carvalho , and P. atragula Pansonato, Mudrek, Veiga-Menoncello, Rossa-Feres, Martins , and Strüssmann (2n = 18); P. ternetzi Miranda-Ribeiro and P. ameghini (Cope) (2n = 20) ( Duarte et al. 2010; Fávero et al. 2011; Cardozo et al. 2016). In addition, Duarte et al. (2010) highlighted that P. florencei sp. nov. has morphological polymorphisms in the chromosomes 7, 8, and 11, which distinguishes it from P. mineira . Moreover, these same authors stated that the two species are distinguished by polymorphisms in the heterochromatin distribution and by the NOR position in the pair 8, which was terminal in P. mineira and subterminal in P. florencei sp. nov..
Pseudopaludicola florencei View in CoL sp. nov. is promptly distinguished from P. canga View in CoL ( Giaretta & Kokubum 2003; Pansonato et al. 2012; Carvalho et al. 2015a), P. giarettai Carvalho (Carvalho 2012 View in CoL ; Carvalho et al. 2015b), P. hyleaustralis Pansonato, Morais, Ávila, Kawashita-Ribeiro, Strüssmann, and Martins (Pansonato et al. 2012) View in CoL , P. facureae View in CoL ( Andrade & Carvalho 2013; Carvalho et al. 2015a), and P. parnaiba Roberto, Cardozo, and Ávila (Roberto et al. 2013 View in CoL ; Carvalho et al. 2015a) by possessing pulsed notes, whereas those species have calls composed of non-pulsed notes. Note structure (three non-concatenated pulses) distinguishes P. florencei View in CoL sp. nov. from species with notes with concatenated pulses (= lack of interpulse interval; sensu Magalhães et al. 2014): P. mystacalis View in CoL [12– 14 concatenated pulses; Pansonato et al. 2013], P. boliviana View in CoL [3–6; Duré et al. 2004], P. ibisoroca Pansonato View in CoL , Veiga- Menoncello, Mudrek, Jansen, Recco-Pimentel, Martins, and Strüssmann [3–12; Pansonato et al. 2016], and P. motorzinho View in CoL [2–6; Pansonato et al. 2016].
The new species is distinguished from other congeners [values within square brackets] with notes with nonconcatenated pulses by the following acoustic traits: P. ternetzi View in CoL has shorter note duration (108–166 [32–80] ms) and interpulse interval (22–92 [1–14] ms), higher note (223–297 [606–921] notes per minute) and pulse rates (18–32 [61–139] pulses per second), and lower peak of dominant frequency (4608–5599 [3516–4500] Hz) ( Andrade et al. 2017b); P. ameghini View in CoL has shorter interpulse interval [1–23 ms], and higher note [348–452 notes per minute] and pulse rates [40–56 pulses per second], and lower peak of dominant frequency [3141–4312 Hz] ( Andrade et al. 2017b); P. atragula View in CoL has longer note duration [300–700 ms], higher number of pulses per note (3–4 [9–36]), lower note rate [42–98 notes per minute], and lower peak of dominant frequency [3618–4264 Hz] ( Pansonato et al. 2014). The three species of the P. saltica View in CoL species group ( P. saltica View in CoL , P. murundu View in CoL , and P. jaredi View in CoL ) vary highly the number of pulses in their notes (2–7, combined values; Andrade et al. 2016); on the other hand, the new species has very stereotyped three-pulsed notes ( Figure 4A View FIGURE 4 ). Pseudopaludicola falcipes View in CoL may have notes with two or three pulses, but those with two pulses are more common (83 %, n = 240 analyzed notes; Figure 4D View FIGURE 4 ). In addition, the new species can be significantly differentiated from P. falcipes View in CoL by having longer note duration and interpulse interval, and lower pulse and note rates (Wilcoxon-Mann-Whitney Test: P <0.01; present study).
In comparison with the two phylogenetically close related species, the new species is distinguished from P. mineira by having stereotyped three-pulsed notes (only two notes of an individual have four pulses; n = 320 analyzed notes), whereas the P. mineira has stereotyped two-pulsed notes ( Figure 4C View FIGURE 4 ). This character and its longer note duration (108–166 [42–87] ms) distinguish the new species from P. mineira ( Pereira & Nascimento 2004; present study). Pseudopaludicola pocoto has also stereotyped three-pulsed notes ( Figure 4B View FIGURE 4 ); however, P. florencei sp. nov. is readily distinguished from P. pocoto by having a higher note rate (223–297 [100–184] notes per minute). Additionally, P. florencei sp. nov. differs significantly from P. mineira by its note rate; and from P. pocoto by having shorter note duration, internote interval and interpulse interval, and higher pulse rate (Exact Wilcoxon- Mann-Whitney Test: P <0.01).
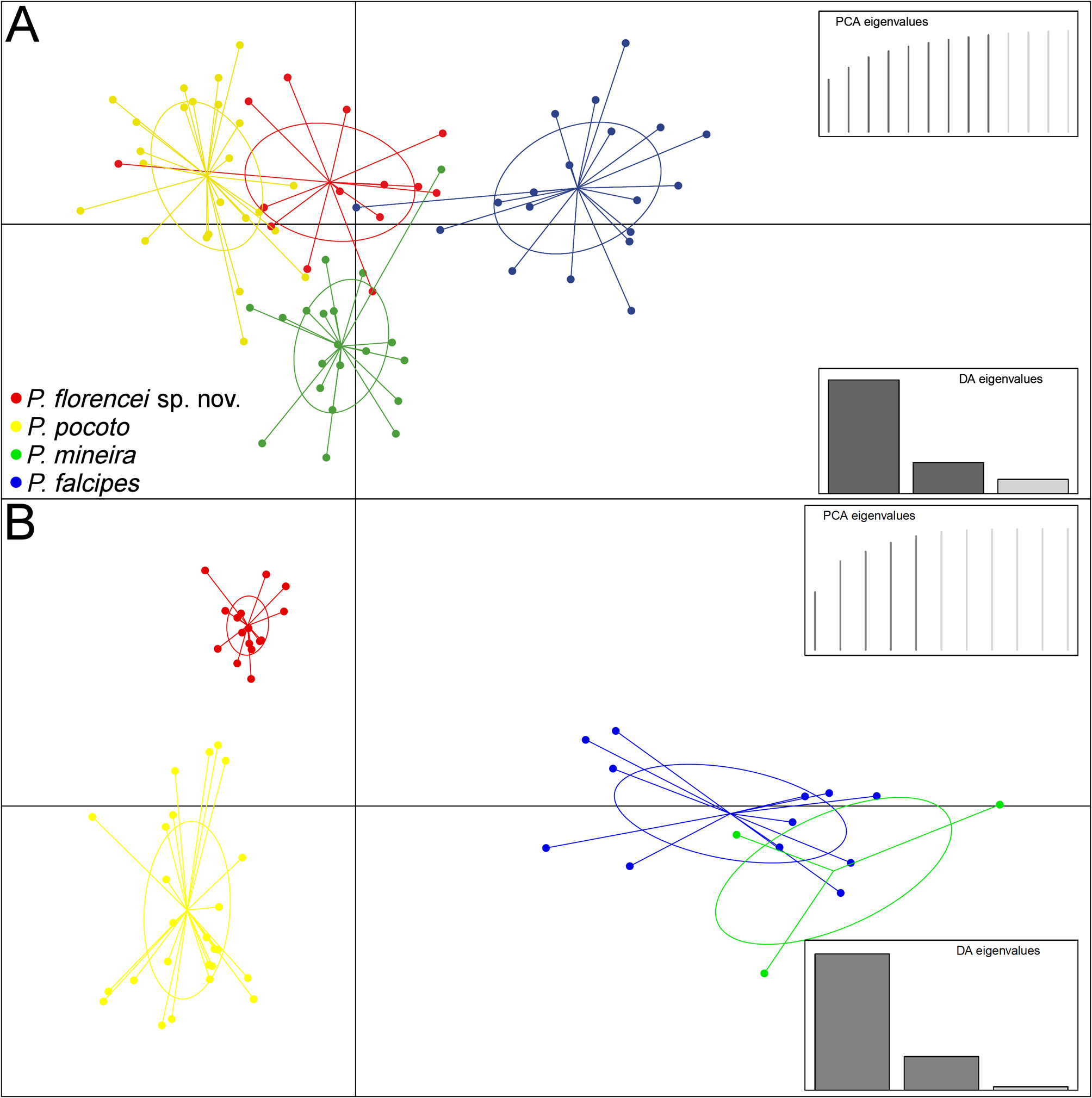
Both multivariate approaches (randomForest and DAPC) to morphometry yielded no noticeable discrimination among species ( Table 3; Figure 5A View FIGURE 5 ). However, the Exact Wilcoxon-Mann-Whitney Test on SVL revealed that P. florencei sp. nov. is larger than P. pocoto , and smaller than P. mineira and P. falcipes ( P <0.01). Regarding calls, the randomForest model resulted in total discrimination among P. florencei sp. nov. and its closer related species ( P. mineira + P. pocoto ) and P. falcipes , with all its individuals classified correctly ( Table 3). The DAPC also revealed substantial differentiation among the new species and its closer related species ( Figure 5B View FIGURE 5 ), with a greater separation along axis 1 (LD1 = 78.6 %; LD2 = 19.4 %). Number of pulses per note (66 %), pulse (12 %), and note (12 %) rates mainly accounted for species separation along LD1 ( Figure 5B View FIGURE 5 ). Number of pulses per note (44 %), internote (27 %), and interpulse (18 %) intervals mainly accounted for species separation along LD2 ( Figure 5B View FIGURE 5 ). See also the similar results presented in the performed PCAs (Appendix 4).
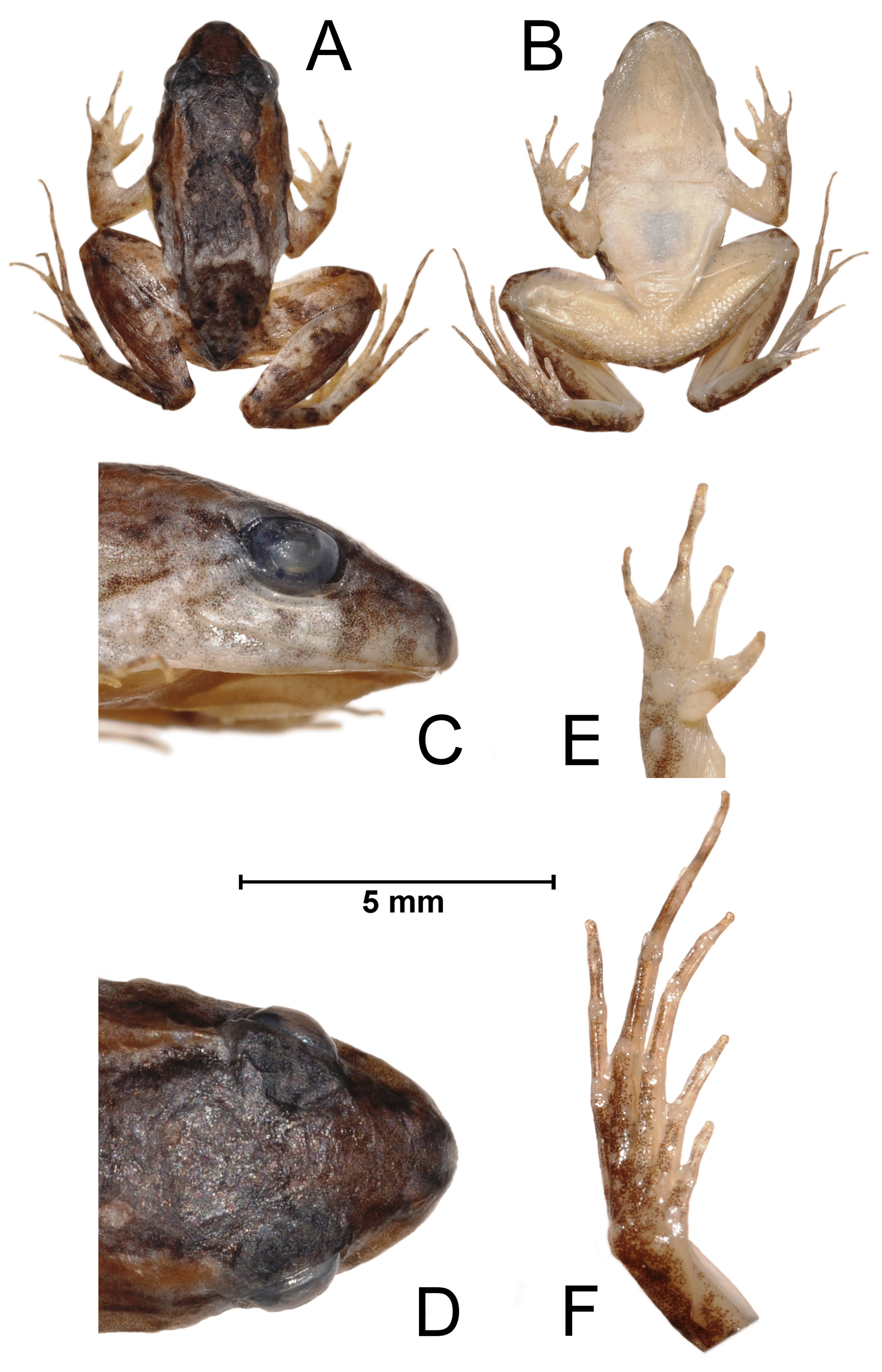
Description of the holotype: Body elliptic and broad ( Figure 2A–B View FIGURE 2 ; Table 1). Head elliptical, slightly wider than long. Snout subovoid in dorsal view and rounded in profile ( Figure 2C–D View FIGURE 2 ). Eye not protuberant. Eye diameter equal to the interorbital distance. Interorbital area flat. Pupil rounded. Upper eyelid without tubercles. Nostril not protuberant and closer to the snout tip than to the eye. Canthus rostralis rounded, smooth. Loreal region slightly concave. Single subgular vocal sac, externally expanded and with discrete longitudinal folds. Choanae rounded well separated from each other. Vocal slits present. Tympanum indistinct. A discrete dermal fold extending from the posterior margin of the eye to the insertion of the arm. Mouth opening ventral. Vomerine teeth absent (unnoticeable also to the touch). Tongue elliptical, longer than wide, free posteriorly, without pigmentation at its base. Flanks with discrete granules. One ovoid antebrachial tubercle present in the first quarter of the forearm and a second ovoid tubercle closer to elbow. Finger and toe tips not expanded. Outer and inner metacarpal tubercles welldefined, outer metacarpal tubercle ovoid and inner metacarpal tubercle elliptical. Fingers with single and rounded subarticular tubercles. Palm of hand smooth, with no supernumerary tubercles. Thumb with a keratinized, light brown nuptial pad, extending from the base of the hand to the proximal limit of the terminal phalanx, covering almost the entire external portion of the finger. Webbing absent between fingers. Relative finger lengths, when adpressed one to another: I <II <IV <III ( Figure 2E View FIGURE 2 ). Outer metatarsal tubercle well defined, conical. Inner metatarsal tubercle elliptical. The internal metatarsal tubercle larger than the external. Toes with well-defined, single, enlarged, and rounded subarticular tubercles. Sole of the foot smooth, with no supernumerary tubercles. Toes webbed basally and fringed along their sides to almost their tips. Fringes developed on all toes (mainly II, III, and IV). External fringe on Toe V continues almost to the outer metatarsal tubercle. Well-developed fold from internal metatarsal tubercle to the mid-ventral tarsus, ending in a tarsal tubercle poor protuberant. Relative toe lengths, when adpressed one to another: I <II <V <III <IV ( Figure 2F View FIGURE 2 ). Hind limb robust and moderately long with the tibio-tarsal articulation just reaching the posterior margins of eye. Thigh shorter than tibia. Foot longer than thigh and tibia. Tubercle absent on calcaneus. Belly skin smooth. Abdominal fold present. Dorsal surfaces of head, body, and limbs smooth. Dorsal surface of body interspersed with some discrete tubercles. Cloacal region smooth ( Figure 2B View FIGURE 2 ). Measurements of the holotype presented in Table 1.
Color pattern of the holotype in preservative. Dorsum grayish with dark grey, white, and brown blotches. Brown dorsolateral stains on body, from posterior corner of eyes to the region of insertion of legs. Belly whitish (unpigmented). Throat whitish, pigmented (with small black dots scattered). Dorsum darker than the dorsal surfaces of limbs. Region between upper lip and eyes with alternating vertical grey and light beige stripes. Ventral faces of arms and legs unpigmented, except of the thigh (pigmented as in throat). Palm of hand pigmented. Sole of foot pigmented and darker than hands, arms, and legs. Color of the sole of the foot similar to that of dorsal leg. Dorsal faces of arms light grayish with dark brown blotches. Dorsal faces of legs light grayish with dark brown transversal discontinuous stripes and with scattered brown blotches. Transverse stripes on thighs (2–3), shanks (3– 4), feet (3–4). Dark brown nuptial pads ( Figure 2A View FIGURE 2 ).
Variation in type series. Dorsal surface of body varies from dark grey to dark brown, with black or dark brown irregular blotches ( Figure 3 View FIGURE 3 ). Ventral surface pigmented (with small black dots scattered) on throat, chest, and belly (ZUEC 23512–3, 23515–8, 23520, 23522–3, 23525–8, 23530). The specimen ZUEC 23520 has a brown vertebral line, and the specimens ZUEC 23512, 23514, and 23520 have white scattered blotches on dorsum. The specimens ZUEC 23512–4, 23516–7, 23519, 23521–5, 23528, and 23530 do not have dorsolateral stains on body, from posterior corner of eyes to the region of insertion of legs. The specimens ZUEC 23514, 23523–4, and 23529– 30 have a discrete tympanic ridge from behind the eyes to the proximal portion of the arms. The specimens ZUEC 23525–8 have white blotches on the region between the mouth corner and the insertion of the arms. The specimen ZUEC 23512 has a large black blotch on dorsum. The specimen ZUEC 23527 has two large black blotches in the posterior portion of the dorsum, slightly above the cloacal region. Females have a more robust body and no nuptial pads, vocal sac and vocal slits.
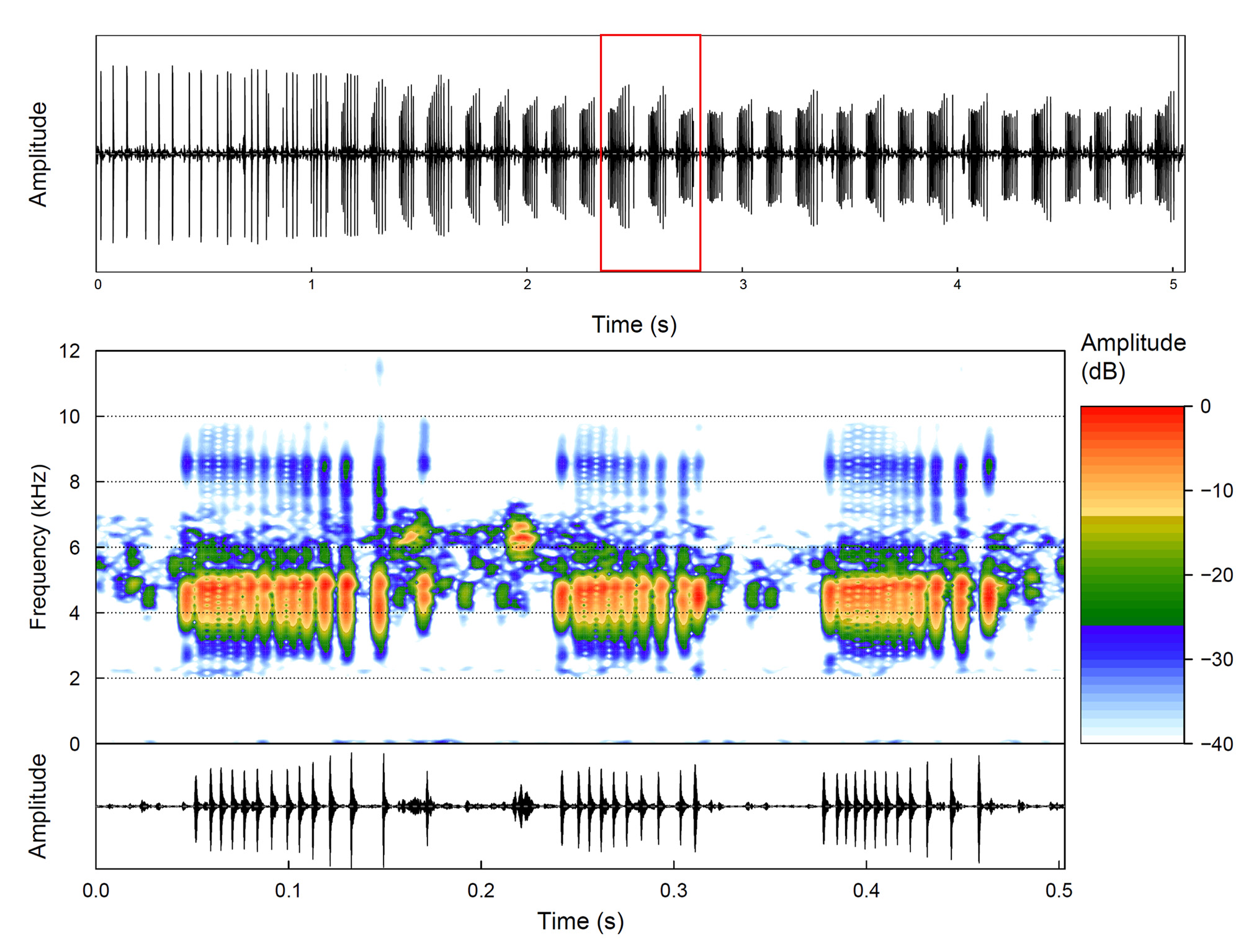
Vocalizations. Pseudopaludicola florencei sp. nov. emits an advertisement call of variable duration that can be long (1.6– 22.4 s), consisting of series of three-pulsed notes (1–5 series of three-pulsed notes per call) that lasts 0.4– 22.4 s, separated by intervals of 103–561 ms ( Figure 4A View FIGURE 4 ). Notes last 108–166 ms separated by intervals of 64–141 ms, and are released at a rate of 223–297 notes per minute; notes have a slightly increase in amplitude from the first to the second pulses (= midpoint of note), followed by a decrease in amplitude regarding the second and third pulses (see oscillogram in Figure 4A View FIGURE 4 ). Notes are composed of three non-concatenated pulses ( Figure 4A View FIGURE 4 ); notes with four pulses were rarely found (only two notes of an individual). Pulses vary from 4–16 ms, separated by intervals of 22–92 ms, and are released at a rate of 18–32 pulses per second ( Figure 4A View FIGURE 4 ). Dominant (= fundamental) frequency peaks are between 4608–5599 Hz; minimum frequency ranges 4005–5125 Hz, and the maximum frequency ranges 4996–6804 Hz. Notes present up to two harmonics; the second ranging from 8915– 10982 Hz (mean = 9834; SD = 525) ( Figure 4A View FIGURE 4 ). Air temperature of recorded calls varied from 22 to 23.5 °C. Call quantitative traits are summarized in Table 2.
An additional pulsed note was noticed shortly after four advertisement calls from two recorded males ( Figure 6 View FIGURE 6 ) and other males heard in the field. Regular notes can gradually gain pulses until they become this note type ( Figure 6 View FIGURE 6 ). This note (n = 10) has a duration ranging from 68–116 ms (mean = 83 ± 16), is composed of 7–14 pulses (mean = 10.9 ± 2.1), lasting 2–9 ms (mean = 5 ± 1), is separated by intervals of 0–22 ms (mean = 4 ± 1), and is emitted at rates of 93–157 pulses per second (mean = 134 ± 22). The dominant frequency varies from 4436–4823 Hz (mean = 4630 ± 158); minimum frequency ranges 3747–3833 Hz (mean = 3781 ± 34) and maximum frequency ranges 4996–5125 Hz (mean = 5060 ± 42) ( Figure 6 View FIGURE 6 ). Another emphasized frequency band may be present, peaking from 8398–8570 Hz (mean = 8488 ± 59).
Distribution. Pseudopaludicola florencei sp. nov. is known from its type locality, and in the municipality of Mutuípe, state of Bahia, Northeastern Brazil, and in the municipality of Nanuque, state of Minas Gerais, Southeastern Brazil (Darío Cardozo pers. Communication; see Figure 1 View FIGURE 1 ). Mutuípe and Nanuque are situated about 200 km southeast and 560 km south of the type locality, respectively.
Natural history. The holotype and paratypes collected on November 2016 were recorded on the banks of a river that crosses the urban area of Andaraí. In this place, we observed hundreds of specimens of P. florencei sp. nov. occurring syntopically with Dendropsophus sp. and Rhinella granulosa. We also have some call recordings of the seven paratypes from Área de Proteção Ambiental Marimbus-Iraquara, which is a protected site.
In the field, we heard and recorded the additional pulsed note abovementioned. In all our field recordings, males were isolated at their calling sites, without any close-range encounters. However, there were dozens of males competing for calling sites at that night; it was possible to observe several males interacting physically. In these interactions, some males jumped quickly over the calling males, but no fighting was observed.
Etymology. The specific name honors Antoine Hercule Romuald Florence. Better known as Hercule Florence, a French artist, painter, polygrapher, and inventor, is acknowledged as the inventor of photography in Brazil in the 19th century. After his return from the Langsdorff’s expedition (from 1826 to 1829), Florence developed a system able to properly describe animal sounds, transcribing them into a five line music staff ( Florence 1831, 1876; Toledo & Araújo 2017). Such method, termed as “Zoophonie” by Florence, was the first universal method of describing animal sounds and he is therefore designated as the “father of bioacoustics” ( Vielliard 1993; Toledo & Araújo 2017). At least these two techniques (photography and zoophony = bioacoustics) are fundamental for species description nowadays ( Köhler et al. 2017). Specifically, bioacoustics has proved to be efficient in clarifying the taxonomy of the genus Pseudopaludicola (as in the present study).
Phylogenetic inferences and mitochondrial DNA divergences. We recovered the same tree topology inferred by Veiga-Menoncello et al. (2014) and Andrade et al. (2016) for the genus Pseudopaludicola (Appendix 5), where species that shows equal diploid chromosome numbers are recovered in well-supported clades. Among species with 2n = 22 chromosomes ( Figure 7 View FIGURE 7 ), we recovered P. florencei sp. nov. as sister group of P. pocoto and
these two as sister group of P. mineira . But this last phylogenetic relationship was recovered with low posterior probabilities, as evidenced in previous studies. The average uncorrected p-distance between P. florencei sp. nov. and P. pocoto was 4.2 % (range 3.4–4.9%; Table 4). The overall genetic distances between all Pseudopaludicola species ranged from 1.8% ( P. canga vs P. sp. from Barreirinhas, state of Maranhão) to 18 % ( P. atragula vs P. boliviana ). The intraspecific distance for P. florencei sp. nov. was 0.1% and it ranged from 0 to 1.5% in other species analyzed ( Table 4).
The advertisement call and acoustic diagnosis of P. falcipes . Quantitative traits are summarized in Table 2. Air temperature of recorded calls varied from 16.0–25.5 °C. Pseudopaludicola falcipes emits a long advertisement calls of variable duration that can be long (7.5– 154.4 s), consisting of series of pulsed notes (1–5 series of notes per call) that lasts 2.8– 110.7 s, separated by intervals of 0.25– 4.4 s ( Figure 4D View FIGURE 4 ). Notes last 32–167 ms separated by intervals of 45–143 ms and are released at a rate of 238–535 notes per minute. Eighty-three percent of all the analyzed notes (n = 320 notes) have two non-concatenated pulses, the other notes with three non-concatenated pulses each. Pulses vary from 4–20 ms, separated by intervals of 15–89 ms, and are released at a rate of 18–62 pulses per second ( Figure 4D View FIGURE 4 ). Dominant (= fundamental) frequency peaks between 4687–5986 Hz; minimum frequency ranges 4219–5599 Hz, and the maximum frequency ranges 4996–6804 Hz. Second harmonic peaks between 8441–11326 Hz (mean = 10188; SD = 666) ( Figure 4D View FIGURE 4 ).
Like the new species, P. falcipes can be distinguished from species that have non-pulsed structure or with concatenated pulses (with lack of interpulse interval): P. canga , P. giarettai , P. hyleaustralis , P. facureae , P. parnaiba , P. mystacalis , P. boliviana , P. ibisoroca , and P. motorzinho . Pseudopaludicola falcipes distinguishes from other congeners [values within square brackets] by the following acoustic traits: P. ternetzi has shorter interpulse interval (15–89 [1–14] ms), higher note (per minute) and pulse rates (238–535 [606–921] notes per minute; 18–62 [61–139] pulses per second), and a lower peak of dominant frequency (4688–5986 [3516–4500] Hz) ( Andrade et al. 2017b); P. ameghini has shorter interpulse interval [1–23 ms] and lower peak of dominant frequency [3141–4312 Hz] ( Andrade et al. 2017b); P. atragula has longer note duration (32–167 [300–700 ms], higher number of pulses per note (2–3 [9–36]), lower note rate [42–98 notes per minute], and lower peak of dominant frequency [3618–4264 Hz] ( Pansonato et al. 2014). From the three long-legged species ( P. saltica , P. murundu , and P. jaredi ), P. falcipes is distinguished by having low variation in the number of pulses per note (2–3) ( Andrade et al. 2016). Pseudopaludicola pocoto has a lower note rate [100–184 notes per minute] (present study). In addition, P. falcipes differs significantly from P. pocoto by its shorter note duration, interpulse and internote intervals, and higher pulse and note rates (Exact Wilcoxon-Mann-Whitney Test: P <0.01). There is no acoustic difference that distinguishes P. falcipes from P. mineira ( Table 3; Figures 4C–D View FIGURE 4 and 5 View FIGURE 5 ).
New distributional data and color variation of P. pocoto . We reported, for the first time, the occurrence of P. pocoto at an altitude of 1545 m in the campo rupestre (or rupestrian grasslands; Silveira et al. 2016) from Pico das Almas, Rio de Contas, Chapada Diamantina (Espinhaço range), state of Bahia. The previous maximum recorded altitude for this species was around 550 m, in Custódia, state of Pernambuco ( Lantyer-Silva et al. 2016). Therefore, this new record extends about 1,000 m the altitudinal distribution of P. pocoto . In the phylogenetic inferences provided by Veiga-Menoncello et al. (2014) and Andrade et al. (2016), there is a specimen (ZUEC 14192; GenBank number KJ147016 View Materials ) from Andaraí recovered together with those of P. pocoto . In our analyzes this specimen was also recovered within P. pocoto . Although we did not hear or collect P. pocoto during fieldwork in Andaraí, it is possible that the two species occur sympatrically in this locality. The three specimens from Pico das Almas were also recovered also within P. pocoto ( Figure 7 View FIGURE 7 ), but with a small genetic distance that might reflect population structure. Until now, we were unable to distinguish this population from the others of P. pocoto based on acoustic comparisons. Therefore, acoustic and genetic evidence support the occurrence of this species in Chapada Diamantina.
Furthermore, we observed that the specimens of P. pocoto from Pico das Almas have a darker color pattern ( Figure 8 View FIGURE 8 ) than that described for the species ( Magalhães et al. 2014). In this population, the dorsal surface varies from dark grey to dark brown, with black or dark brown irregular blotches. Ventral surface varies from whitish to light beige, highly pigmented. The pigmentation on ventral surface may be present on throat, chest, and belly or still may be absent. Vertebral line may be absent, being present on only six males. Some specimens have enlarged white blotches on dorsum and/or dorsal surface of hind limbs, and white blotches on the region between the mouth corner and the insertion of the arms.
| CFBH |
Universidade Estadual Paulista |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Leiuperinae |
|
Genus |
Pseudopaludicola florencei
| Andrade, Felipe Silva De, Haga, Isabelle Aquemi, Lyra, Mariana Lúcio, Leite, Felipe Sá Fortes, Kwet, Axel, Haddad, Célio Fernando Baptista, Toledo, Luís Felipe & Giaretta, Ariovaldo Antonio 2018 |
Pseudopaludicola
| Andrade & Haga & Lyra & Leite & Kwet & Haddad & Toledo & Giaretta 2018 |
Pseudopaludicola
| Andrade & Haga & Lyra & Leite & Kwet & Haddad & Toledo & Giaretta 2018 |
Pseudopaludicola florencei
| Andrade & Haga & Lyra & Leite & Kwet & Haddad & Toledo & Giaretta 2018 |
P. florencei
| Andrade & Haga & Lyra & Leite & Kwet & Haddad & Toledo & Giaretta 2018 |
P. parnaiba
| Roberto, Cardozo 2013 |
P. giarettai
| Carvalho (Carvalho 2012 |
P. hyleaustralis Pansonato, Morais, Ávila, Kawashita-Ribeiro, Strüssmann, and Martins (Pansonato et al . 2012)
| Pansonato, Morais, Avila, Kawashita-Ribeiro, Strussmann 2012 |
P. canga
| Giaretta and Kokubum 2003 |
P. murundu
| Toledo, Siqueira, Duarte, Veiga-Menoncello, Recco-Pimentel 1957 |
P. ternetzi
| Miranda-Ribeiro 1937 |
P. boliviana
| Parker 1927 |