Catocala nilssoni, Saldaitis, Aidas, Pekarsky, Oleg & Borth, Robert J., 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4254.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:8D08D2CC-A6CD-44B2-B694-93202C239BC4 |
|
DOI |
https://doi.org/10.5281/zenodo.6025130 |
|
persistent identifier |
https://treatment.plazi.org/id/58885DB8-1BE3-43D9-BED7-D8CABBDE66F8 |
|
taxon LSID |
lsid:zoobank.org:act:58885DB8-1BE3-43D9-BED7-D8CABBDE66F8 |
|
treatment provided by |
Plazi |
|
scientific name |
Catocala nilssoni |
| status |
sp. nov. |
Catocala nilssoni sp. n.
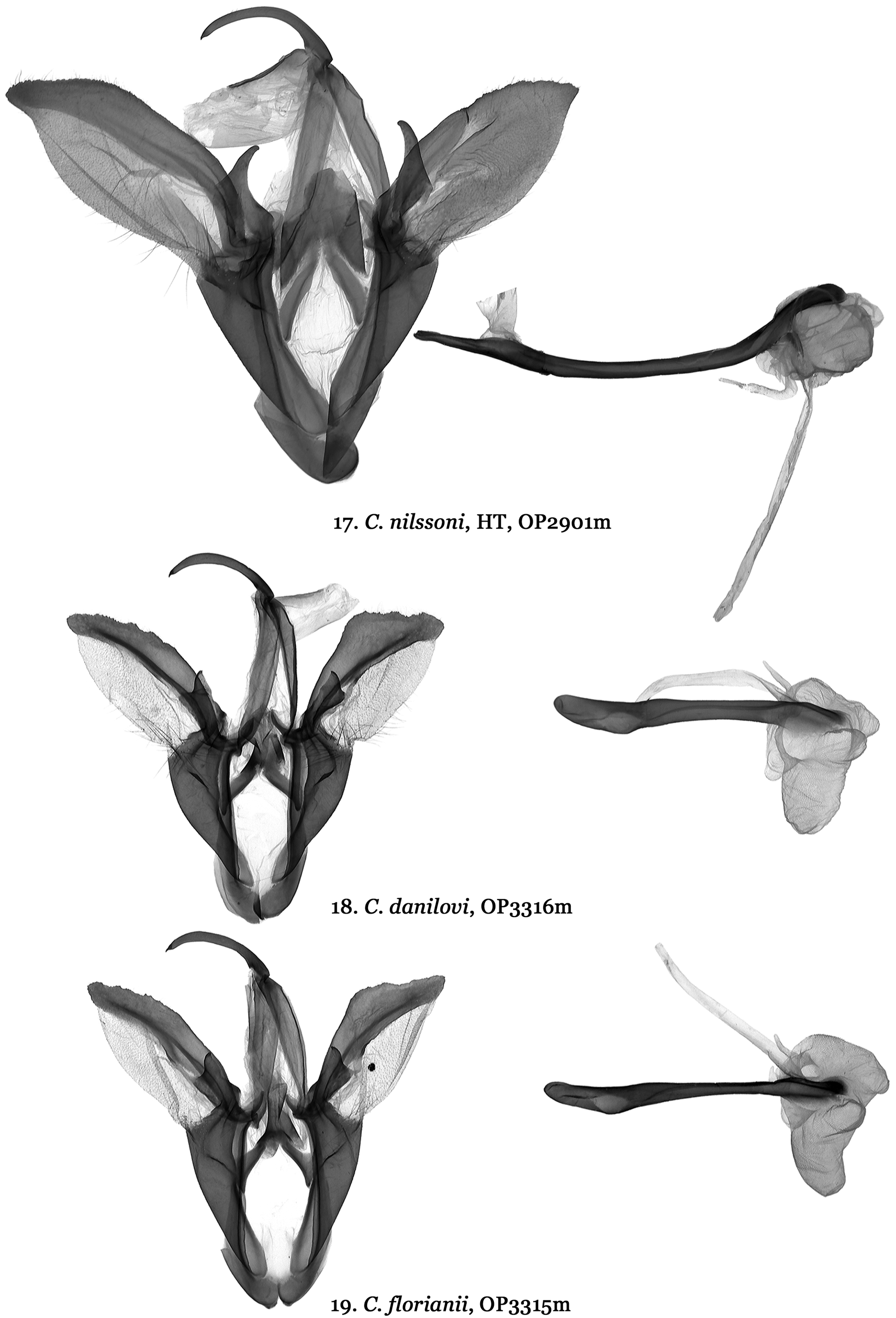
( Figs 1, 2 View FIGURES 1 – 8 , 17 View FIGURES 17 – 19 , 27 View FIGURES 27 – 30 )
Type material. Holotype: male ( Fig. 1 View FIGURES 1 – 8 ), China, prov. Liaoning, Da-gu-Shan, Gushan zhen, n. Donggang , 1- 11.vii.2013, leg. Li Jingke, slide No. OP 2901m (coll. PGM, later to be deposited in the HNHM).
Paratype: female ( Fig. 2 View FIGURES 1 – 8 ), China, Shandong province, Mount Laoshan , 1000 m, Qingdao City, NL 36’20, EL 120’80, 08–24.vii.2007, leg. Li Jingke, slide No. OP 2722f (JB1864f), DNA No. 10255 - 130707 - CH (coll. DNK).
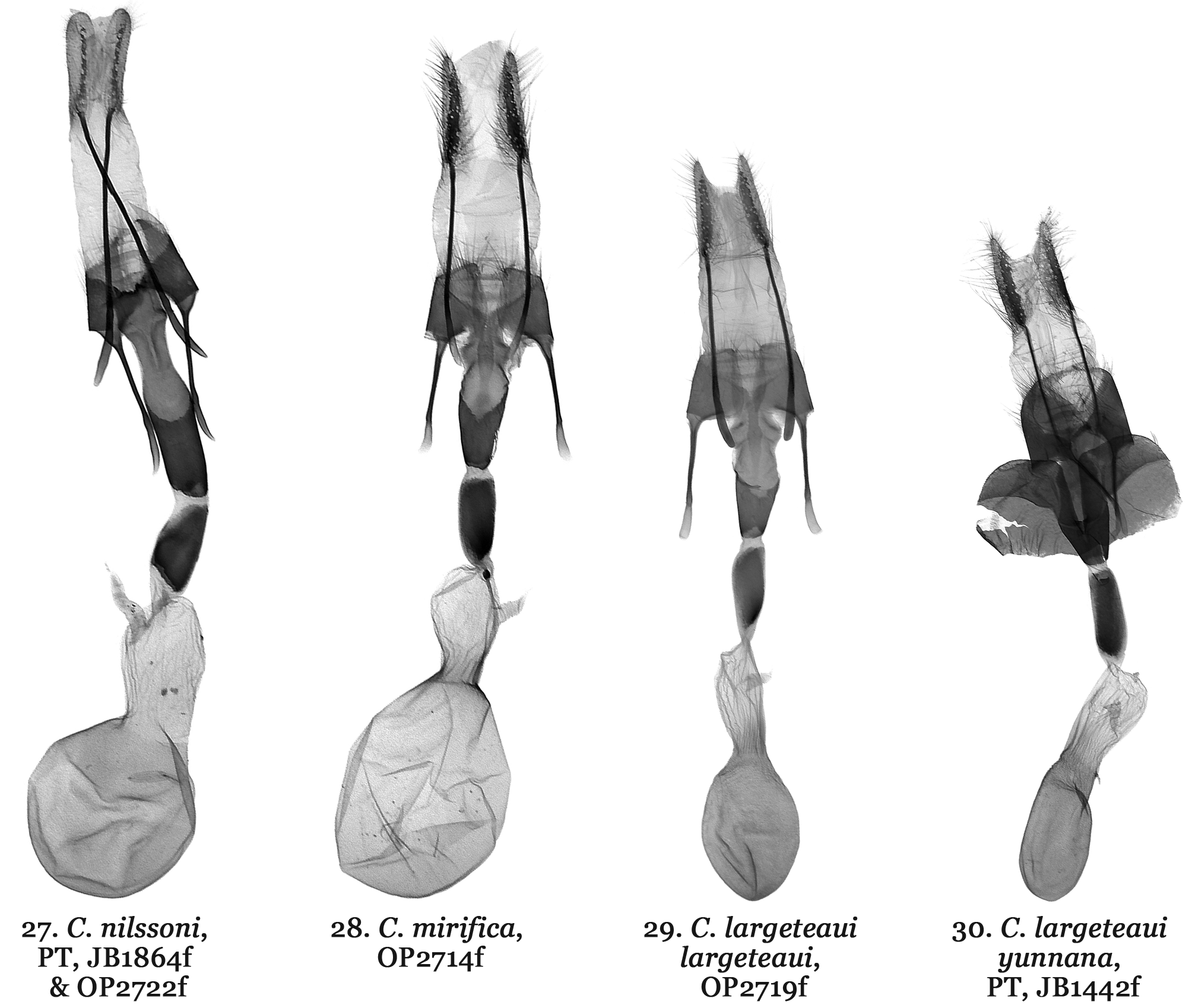
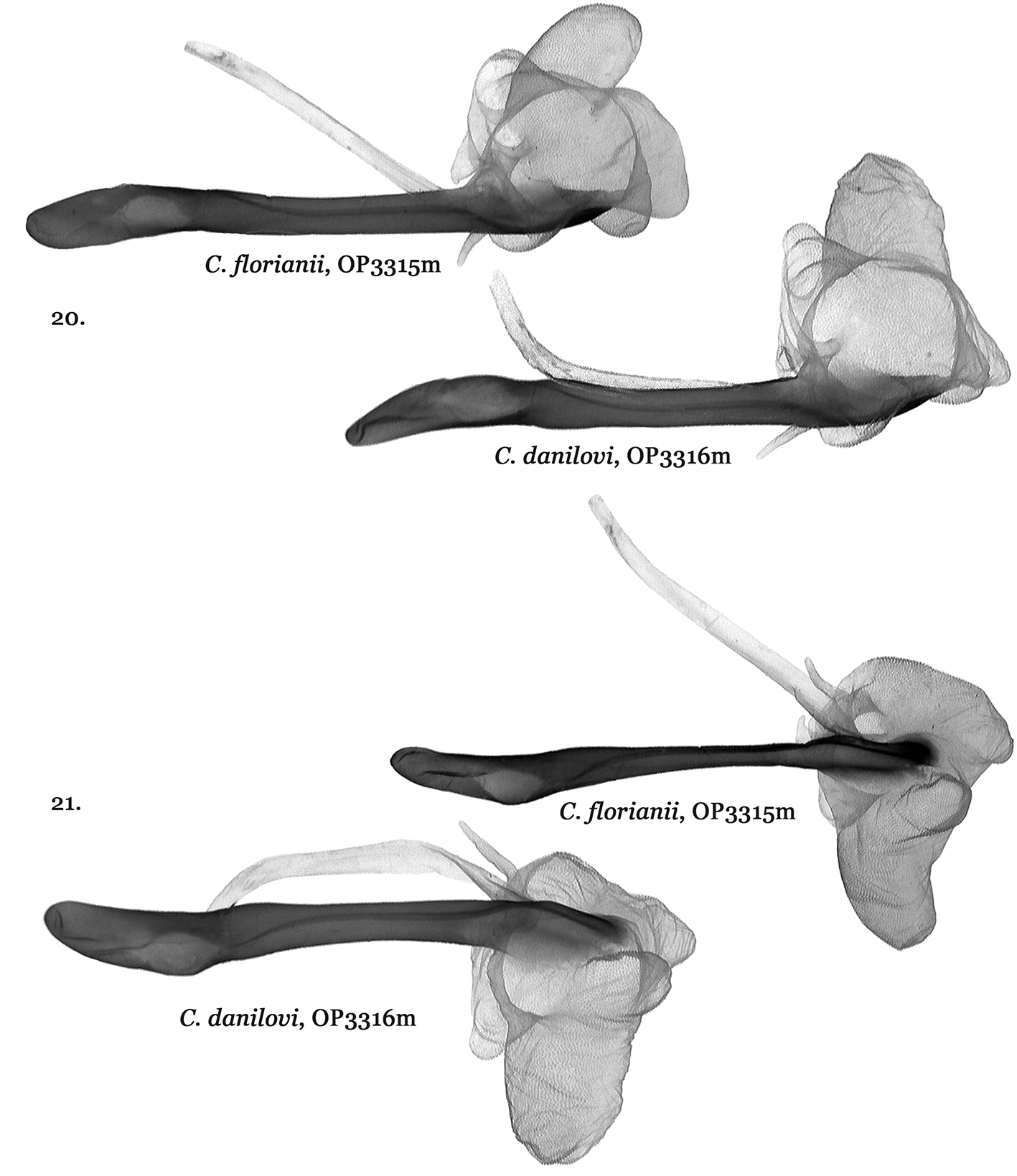
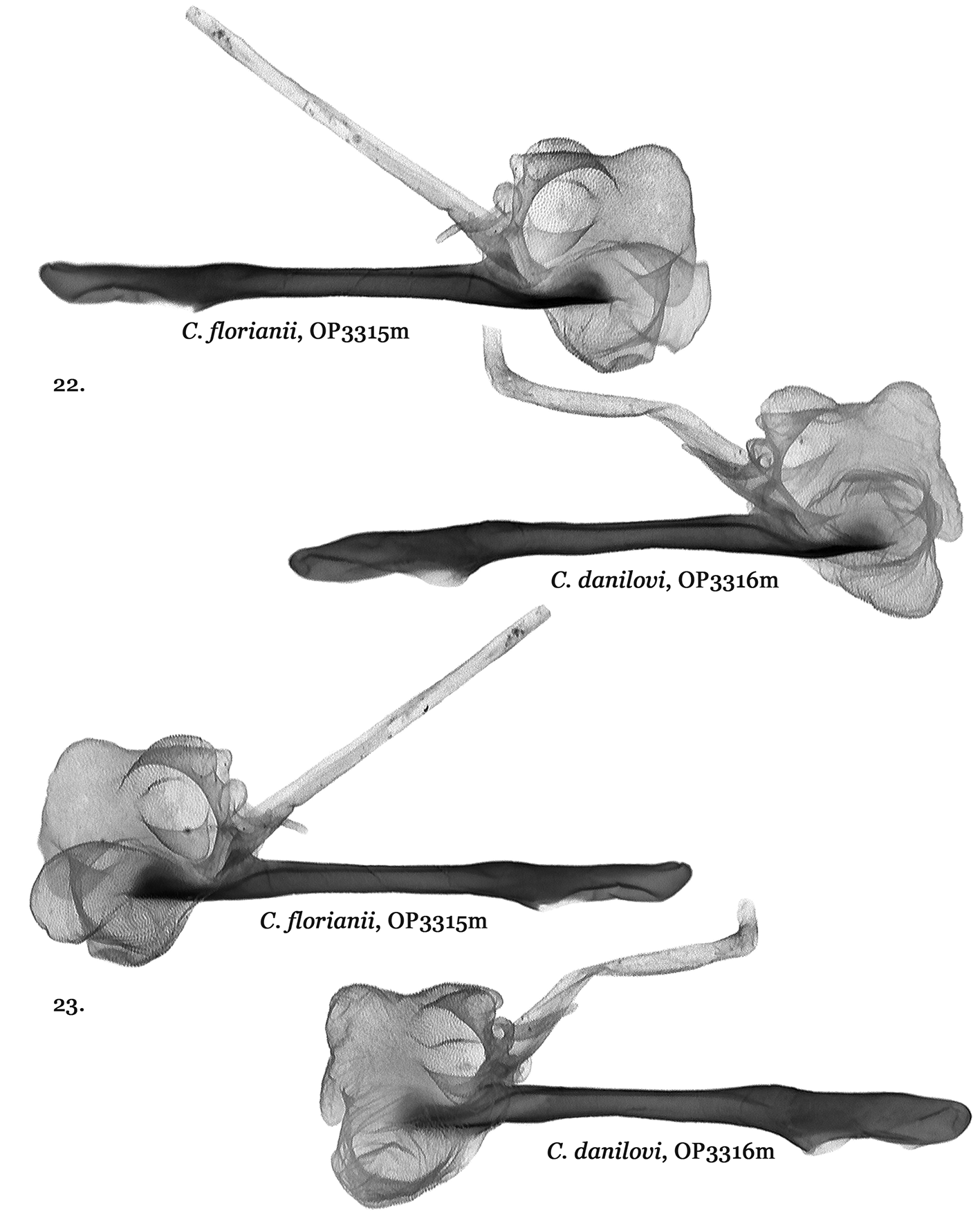
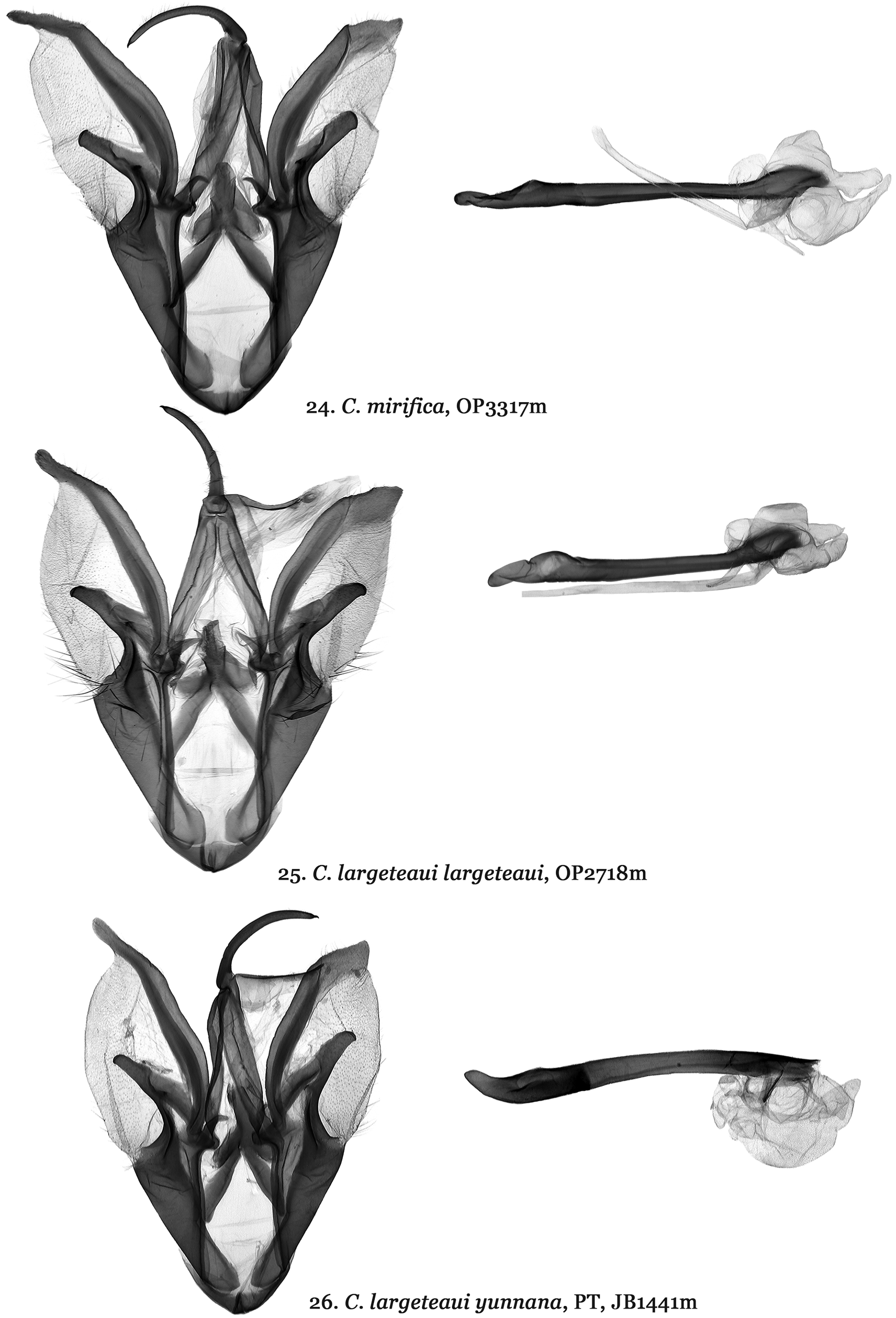
Diagnosis. Catocala nilssoni ( Figs 1, 2 View FIGURES 1 – 8 ) differs from all other Catocala species and does not appear to belong to any known Catocala species group. Based on a combination of wing pattern and DNA it appears most similar to the C. danilovi-florianii species group and the C. mirifica Butler, 1877 species ( Figs 9–16 View FIGURES 9 – 16 ) complex. The new species is the only Palearctic Catocala whose forewings have a broad black patch extending from the inner margin to CU2 and from the antemedial area to the postmedian line. This ground pattern appears in Catocala alabamae Grote, 1875 which has a similar forewing patch in its rare form olivia H. Edwards, 1880 ( Fig. 8 View FIGURES 1 – 8 ) which has shown up twice in a reared brood of otherwise nominative C. alabamae (Jeff Slotten pers. com. 2005). This large dark patch also shows up in Catocala minuta Edwards W.H., 1865 (= parvula W. H. Edwards, 1865) and with only two known specimens of C. nilssoni it remains uncertain whether this patch is present in all individuals. In C. mirifica ( Figs 9, 10 View FIGURES 9 – 16 ) and Catocala largeteaui Oberthür, 1881 ( Figs 11–14 View FIGURES 9 – 16 ) the black area is only in the apical part of the forewings. Catocala nilssoni also differs from C. mirifica and C. largeteaui by the wider and shallower loop formed by its hind wing median band. The new species is significantly larger than C. danilovi (wingspan of 49-56 mm vs. 42–43 mm) ( Figs 3, 4 View FIGURES 1 – 8 ) and has forewings with a pale brown background compared to the teal-grey in C. danilovi . The hind wings of C. nilssoni have a broader orange-yellow area as in C. danilovi . New species male genitalia ( Fig. 17 View FIGURES 17 – 19 ) differ from those of C. danilovi ( Figs 18 View FIGURES 17 – 19 , 20–23 View FIGURES 20 – 21 View FIGURES 22 – 23 ) and C. florianii ( Figs19 View FIGURES 17 – 19 , 20–23 View FIGURES 20 – 21 View FIGURES 22 – 23 ) by being more symmetrical (valvae similar in size and in shape), much larger in size with smooth costal margin of valva, larger, elongated harpe and longer, curved aedeagus, whereas genitalia of C. danilovi and C. florianii characterized by smaller size, marked asymmetry (left valva narrower with dentate costal margin, right valva larger with humped costal margin), shorter harpe with concave apex and shorter, almost straight aedeagus. Catocala nilssoni male genitalia differ considerably from those of the C. mirifica species group ( Figs 24–26 View FIGURES 24 – 26 ) in size, form and structure of the valvae, harpe, aedeagus and especially the vesical. New species male genitalia considerably larger in size than male genitalia of C. mirifica species group, costal sclerotization of the left valva is wide, its apex acute, harpe tapering with elongated, narrow tips, aedeagus curved, vesica globular, male genitalia of C. mirifica species group valval costa noticeably narrower with digitiform apical extension, tough, thick, bar-like harpe with blind tips, straight aedeagus, irregular shaped vesica with two large, elongated medial diverticula. Female genitalia of the C. nilssoni ( Fig. 27 View FIGURES 27 – 30 ) are similar to C. mirifica species group ( Figs 28–30 View FIGURES 27 – 30 ) but noticeably larger in size with longer antrum, which is constricted posteriorly, its anterior part with parallel edges, whereas C. mirifica species has shorter antrum with conical anterior part of antrum.
Description. Forewing length of holotype male 22 mm, wingspan 49 mm; forewing length of paratype female 26 mm, wingspan 56mm. Head, collar brown-grey, tegulae light grey, abdomen grey with yellow hair-like scales. Grey-brown forewings with mostly indistinct markings other than a broad black patch extending from the antemedial area to the postmedian line and from above the inner margin to CU2 with darker brown shading extending distally from the patch to the outer margin and also at the apex. Faint brown antemedial line forming convex loop above the patch; median line diffused and dark brown from the costal margin to the anterior edge of the two faint concentric circles forming the reniform; postmedial line sharp and black extending obliquely to most distally pointing tooth between veins M1 and M2 becoming pale but still discernible at second most distal tooth between veins M2 and M3 down to inner margin; subterminal line pale and diffuse becoming darker at last two undulations above the margin. Area between postmedial and subterminal lines richer brown distal to the black patch. Hindwings orange-yellow; median black hindwing band forming unconstricted loop from wing base but bulging between veins M2 and CuA1, terminal band broad and even, nearly broken at tornus; prominent orangeyellow apical patch; fringe orange-yellow with black patches at ends of veins. Underside of forewings greyish cream; basal and terminal fields greyish black; postmedian fascia intensive black. Underside of hindwings in basal field yellow; median band not forming visible loop, reaching dorsum; edge of dorsum blackish yellow; subterminal band without full disjunction in tornal edge. Male genitalia ( Fig. 17 View FIGURES 17 – 19 ). Uncus relatively short, narrow, evenly curved, apically with fine hook; tegumen elongated; juxta sclerotized, double elongated segments; vinculum with strong saccus; valvae slightly asymmetrical, oval-shaped with acute apex; costal margins heavily sclerotized with upper part slightly serrated. The sclerotization of the costal margin of left valva is wider than on the right valva, and runs along the full length, whereas the sclerotization on the right valve is narrower, present only in proximal part and reaches roughly the middle of the valva. Harpe large and wide at the base, tapering with an elongated, evenly curved apical part; aedeagus strongly sclerotized, elongated, cylindrical, curved; caecum elongated; carinal plate massive, strong, evenly curved; vesica membranous, main part globular, multidiverticulate, distal tube long. Female genitalia ( Fig. 27 View FIGURES 27 – 30 ). Ovipositor large, elongated, relatively narrow; papilla analis elongated, broadly rounded at apex and oval shaped, hairy with thin, short seta; apophyses posteriors strong, long; apophyses anteriores shorter than apophyses posteriores; ostium bursae as wide as antrum; antrum long, heavily sclerotized, cylindrical with parallel margins, constricted posteriorly; ductus bursae short, heavily sclerotized, tapering; corpus bursae membranous with cylindrical upper part and globular main part.
Molecular analysis. DNA barcoding results based on percentage differences with one specimen of C. nilssoni appear consistent with the morphology. Full length 658 base pair sequences of the Cytochrome Oxidase Subunit 5' Region (CO1–5P) gene were prepared by the University of Guelph's Barcode of Life Data Systems (BOLD) by methods described in Hebert et al. (2003). Molecular variation based on the Kimura two-parameter distance model for COI DNA barcodes between a single specimen of C. nilssoni follow: two C. danilovi 3.94%; two C. florianii at least 4.11%; six Catocala largeteaui yunnana ( Mell, 1936) at least 3.97%; nine C. largeteaui 4.42%; two C. mirifica at least 4.75%. The specimen of C.nilssoni had the following sequence for COI 5‘positions 1 to 658: AACTTTATATTTTATTTTTGGGATTTGAGCAGGAATAGTAGGAACTTCATTAAGATTATT
AATTCGAGCTGAATTAGGTAATCCTGGATCTTTAATTGGAGATGATCAAATTTATAATAC
TATTGTTACAGCTCATGCTTTTATTATAATTTTTTTTATAGTTATACCAATTATAATTGG
AGGATTTGGTAATTGATTAGTACCTTTAATATTAGGAGCTCCTGATATAGCTTTCCCTCG
TATAAATAATATAAGTTTTTGACTTCTACCCCCCTCATTAACTTTACTAATTTCGAGAAG
AATTGTAGAAAACGGAGCAGGAACTGGATGAACAGTTTATCCCCCCCTTTCTTCTAACAT
TGCTCATAGAGGAAGTTCAGTAGATTTAGCTATTTTTTCTTTACACTTAGCTGGAATTTC
ATCAATTCTAGGAGCTATTAACTTTATTACTACAATTATTAATATACGATTAAATAATTT
AATATTTGATCAAATACCTTTATTTGTTTGAGCTGTAGGAATTACTGCATTCCTTCTTCT
TCTTTCATTACCAGTATTAGCCGGAGCTATTACTATACTTTTAACTGATCGAAATTTAAA
TACTTCTTTTTTTGACCCTGCTGGAGGAGGAGATCCTATTTTATATCAACATTTATTT
Biology and distribution. Both specimens of C. nilssoni were collected in July at light in the mountains of northeast China. In 2007 in Shandong Province a female was found at an elevation of 1000 meters on Mount Lao and in 2013 a male was collected in Liaoning Province in the Da-gu Mountains.
Etymology. The new species is named after Danny Nilsson (Kalvehave, Denmark) for his obsession with entomology and providing the first specimen for our study.
Remarks. While the new species clearly differs from the C. mirifica species group it is noted that the C. mirifica species group remains largely unresolved. We sequenced 18 individuals from this group obtaining 8 different haplotypes within 5 groups with sequences varying by no more than one base pair. Catocala largeteaui differs in COI from the C. mirifica by only 0.62% and was hypothesized by Ishizuka (1982) to be a subspecies of C. mirifica . A greater 3.3% COI variation occurs between C. largeteaui and its subspecies, C. largeteaui yunnana ( Figs 15, 16 View FIGURES 9 – 16 , 26 View FIGURES 24 – 26 , 30 View FIGURES 27 – 30 ) which has a uniformly dark forewing unlike the pale forewing with contrasting dark apical region of C. largeteaui ( Figs 11–14 View FIGURES 9 – 16 ). While little variation was observed in the female genitalia some potential male genitalia differences in the anterior rosette of the vesica exist between C. largeteaui and its subspecies but more dissections of sequenced specimens are needed to confirm these differences and better determine how many valid species are present in the mirifica group.
Catocala danilovi ( Figs 3, 4 View FIGURES 1 – 8 , 18 View FIGURES 17 – 19 , 20–23 View FIGURES 20 – 21 View FIGURES 22 – 23 ) and C. florianii ( Figs 5–7 View FIGURES 1 – 8 , 19 View FIGURES 17 – 19 , 20–23 View FIGURES 20 – 21 View FIGURES 22 – 23 ) do have male genitalia differences but not those diagnosed in the original C. florianii description. When C. florianii was described in 2008 authors Saldaitis & Ivinskis, lacking specimens of C. danilovi , used C. danilovi male genitalia figures from Park et al. 2006 for their diagnosis. Upon the authors obtaining and dissecting a C. danilovi male specimen it became clear that the Park et al. (the same genital picture was used in Kononenko, 2010) C. danilovi genitalia figure relied upon was instead Catocala moltrechti O. Bang-Haas, 1927 . While similar the Catocala danilovi ( Figs 18 View FIGURES 17 – 19 , 20–23 View FIGURES 20 – 21 View FIGURES 22 – 23 ) male genitalia differ from those of C. florianii ( Figs 19 View FIGURES 17 – 19 , 20–23 View FIGURES 20 – 21 View FIGURES 22 – 23 ) by the form of the right valva costa in addition to the cuculus, harpae, aedeagus and especially the vesica structure.
The C. danilovi holotype figured in Park et al. 2006 was also incorrect. The C. danilovi holotype ( Fig. 3 View FIGURES 1 – 8 ) is instead shown in Kononenko (2010: Plate 12, Fig. 7 View FIGURES 1 – 8 ) where the male is the holotype rather than the female shown in Fig. 8 View FIGURES 1 – 8 .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Catocala nilssoni
| Saldaitis, Aidas, Pekarsky, Oleg & Borth, Robert J. 2017 |
Catocala moltrechti
| O. Bang-Haas 1927 |