Alkindus Distant, 1889
|
publication ID |
https://doi.org/10.11646/zootaxa.3750.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:FB494D60-ADD0-4375-B7A8-8F35AB1B5495 |
|
persistent identifier |
https://treatment.plazi.org/id/DD5487B1-7842-AE58-FF2A-FDCB95B85AF0 |
|
treatment provided by |
Plazi |
|
scientific name |
Alkindus Distant, 1889 |
| status |
|
Type species: Alkindus atratus Distant , by monotypy.
Alkindus Distant, 1889: 309 ; Lethierry & Severin 1893: 14 (catalogue); Horvath 1919: 231; McAtee & Malloch 1933: 347–348 (redescription).
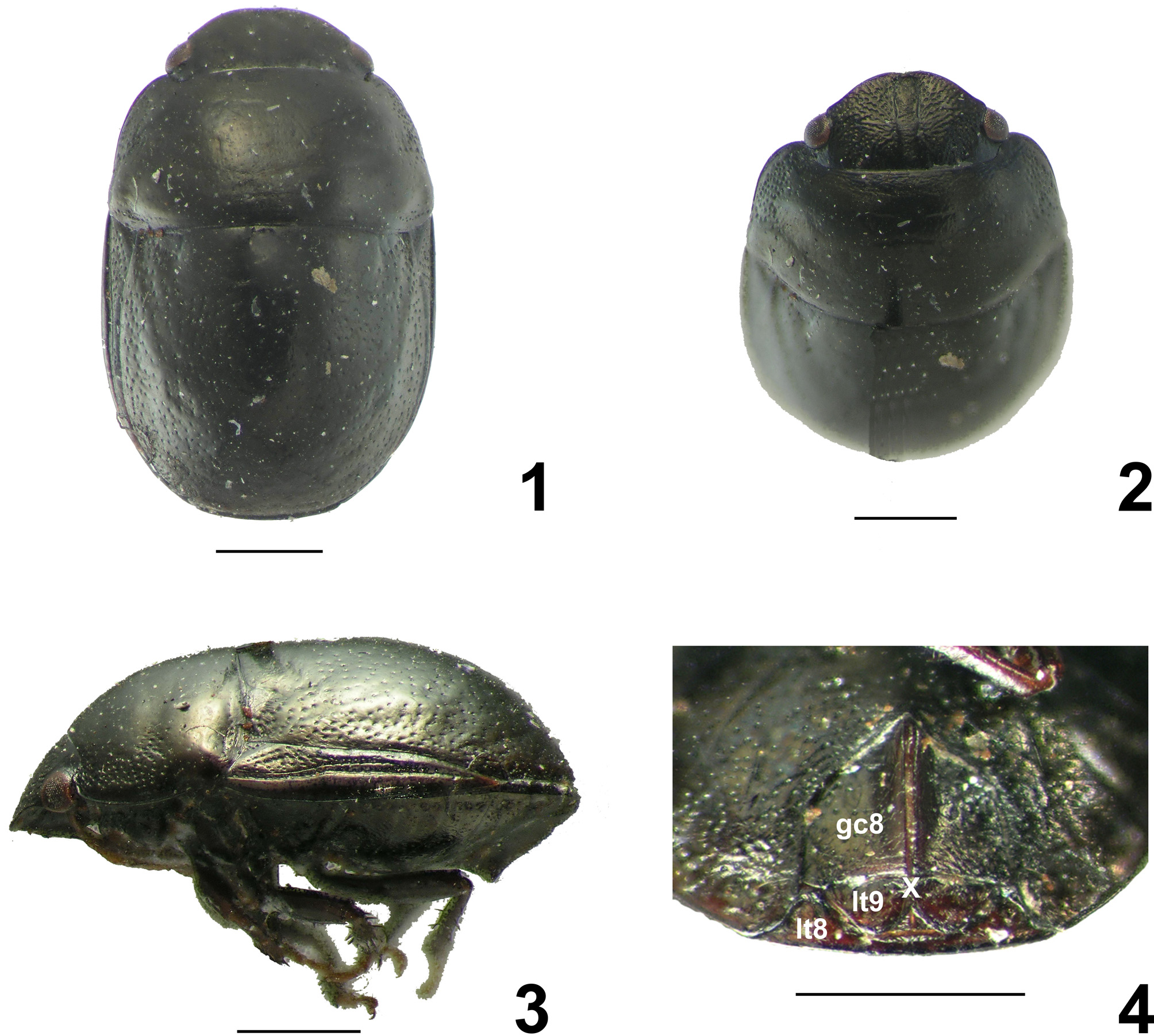
Description. Ovoid ( Fig. 1 View FIGURES 1 – 4. A ), dorsally convex ( Fig. 3 View FIGURES 1 – 4. A ), about 1.5 times longer than wide.
Coloration. Black, shining; eyes yellowish brown to reddish brown; antennae yellowish ocher, sometimes infuscated; rostrum dark brown to ocher; legs dark brown to black, except for the tarsi, yellowish ocher, sometimes infuscated; venter black, except for abdominal sternites IV to VII sometimes with reddish brown lateral stripes.
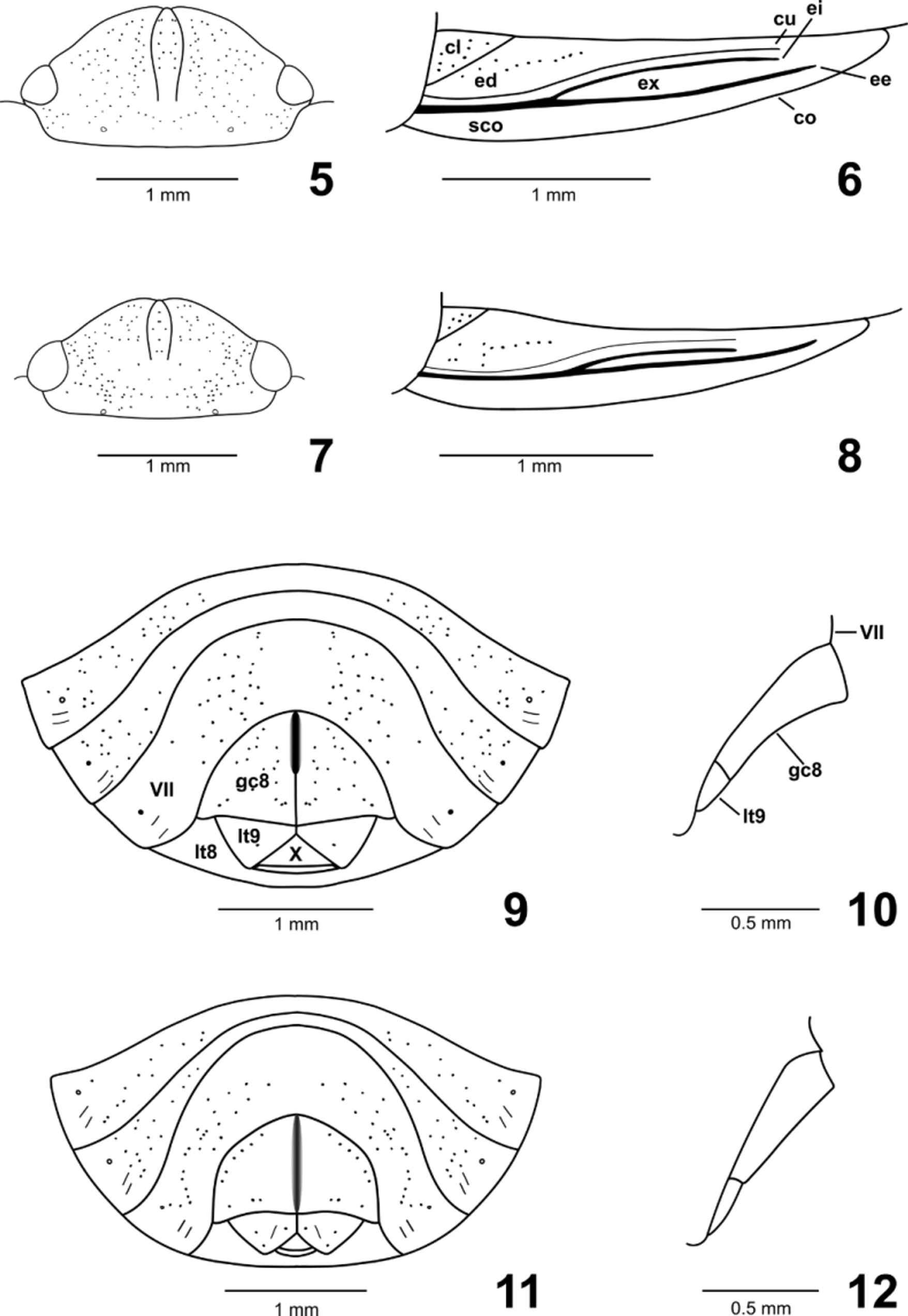
Head ( Figs. 2 View FIGURES 1 – 4. A , 5, 7 View FIGURES 5–12 ). Subtrapezoidal, more than two times wider than long, declivent; densely and deeply punctate, especially on anterior and lateral areas; clypeus nearly impunctate, except subapically. Mandibular plates slightly longer than clypeus, convergent at apex, not covering completely the apex of clypeus; external margins broadly convex, slightly deflected and emarginated. Maxillary plate concave between eye and rostrum; rostrum reaching mid coxae.
Pronotum ( Figs. 1–3 View FIGURES 1 – 4. A ). Subrectangular. Disc with small and shallow punctures, sometimes inconspicuous; lateral area strongly punctate, punctures denser and deeper than on disc. Lateral margins emarginate. Humeral angles broadly rounded; posterolateral angles tumid and rounded.
Scutellum ( Figs. 1, 3 View FIGURES 1 – 4. A ). Base slightly depressed laterally; apex rounded, clearly surpassing posterior margin of tergite VII in both sexes, almost reaching apex of abdomen, covering lateral part of abdomen and the membrane of hemelytra. Disc impunctate, surrounded by dense and regularly distributed punctures, smaller and denser near lateral and posterior margins.
Hemelytra ( Figs. 3 View FIGURES 1 – 4. A , 6, 8 View FIGURES 5–12 ). Corium narrow, acute at apex. Punctures at clavus, endocorium, and along veins; punctures at costa smaller and shallower. Costa emarginated, declivent, smooth and shiny. Exocorial vein bifurcated from middle third, strongly impressed from the base, forming a shallow groove along its external branch. Cubital vein deeply impressed from the base. Corium usually reaching base of abdominal segment VI.
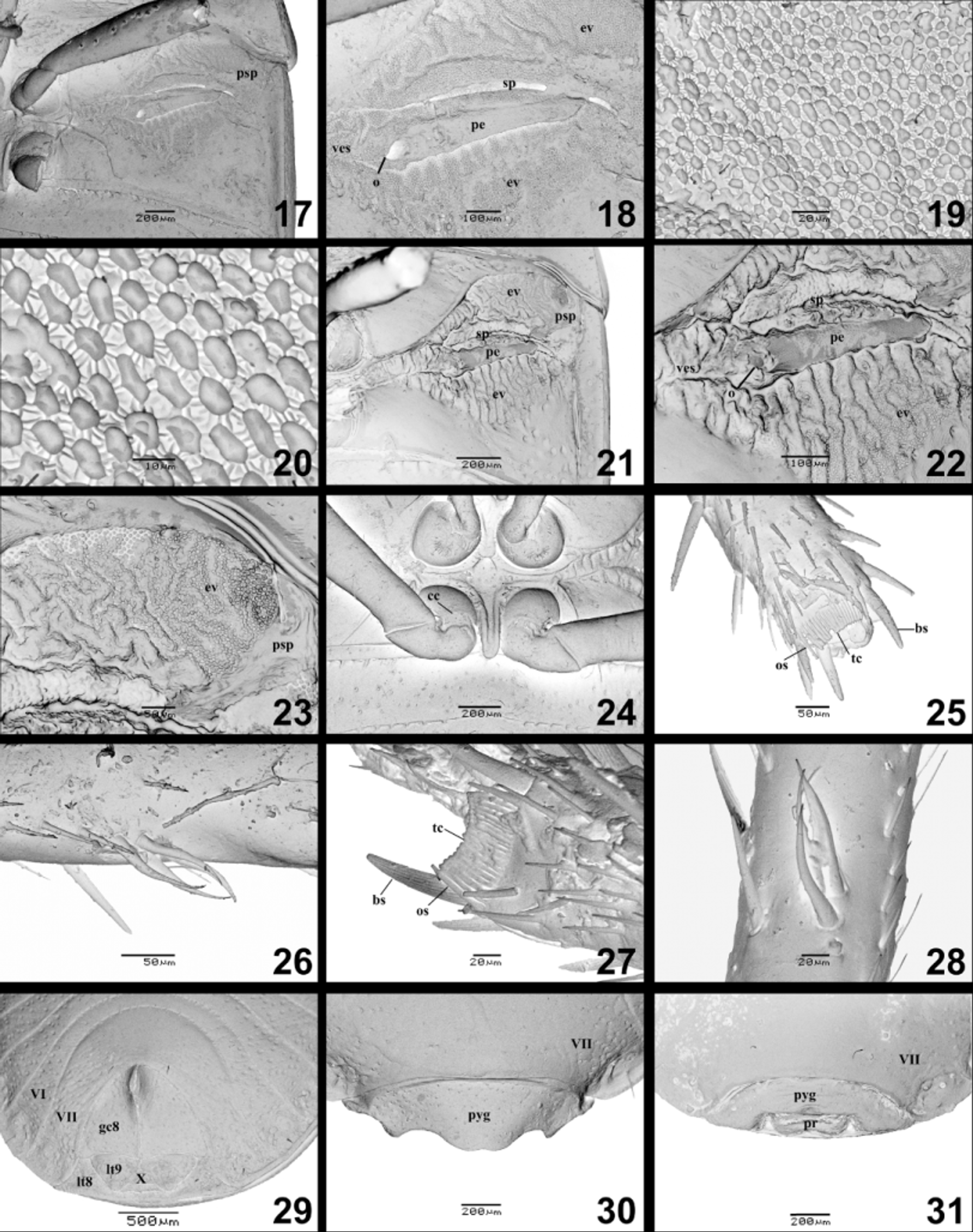
Thoracic venter ( Figs. 17–28 View FIGURES 17–31 ). Anterior half of propleura strongly punctate, posterior half less punctate and concave; prosternal median sulcus moderately deep, margins deflected and more widely separated anteriorly. Anterior half of mesopleura polished and strongly punctate, mesopleural evaporatorium not reaching anterior margin of the segment, macrosculpture showing gyrification ( Figs. 17, 18, 21, 23 View FIGURES 17–31 ) mycoid microsculpture with mushroom-like bodies; pseudoperitreme present ( Figs. 17, 21, 23 View FIGURES 17–31 ); mesosternal median carina shallow, with a small posterior projection. Metapleural evaporatorium not reaching lateral margin of the segment, macrosculpture showing gyrification ( Figs. 17, 18, 21, 22 View FIGURES 17–31 ) and mycoid microsculpture with mushroom-like bodies ( Figs. 19, 20 View FIGURES 17–31 ); vestibular scar present ( Figs. 17, 21 View FIGURES 17–31 ), peritremal groove covering 3/4 of the segment width; peritremal surface nearly smooth ( Figs. 18, 22 View FIGURES 17–31 ); posterior half of metapleura polished, sparsely punctate; metasternum with small projection between coxae, posteriorly directed and medially sulcated ( Fig. 24 View FIGURES 17–31 ). Coxal combs present on all legs ( Fig. 24 View FIGURES 17–31 ); foretibial combs present, consisting of a row of 17 setae, two outer setae only slightly longer than the remaining ones, basal longitudinally ridged spines placed slightly posteriorly to the line of tibial comb, tibial fossula hardly distinguishable, surrounded by pilosity ( Figs. 25, 27 View FIGURES 17–31 ); foretibial apparatus present, consisting of four setae ( Figs. 26, 28 View FIGURES 17–31 ). All tibiae flattened dorsally; hind tibiae scarcely sulcate.
Abdominal venter ( Figs. 9, 11 View FIGURES 5–12 , 13, 15 View FIGURES 13–16 ). Sternites IV and especially V and VI medially constricted; punctures sparse on all segments, denser laterally, median area impunctate; paired trichobothia at sternites III–VII, longitudinally arranged, although slightly oblique, with the anterior trichobothrium innermost; spiracles nearer to the trichobothria than to the lateral margins of sternites; sometimeswith single long paramedian bristle on each side of sternite VII in the male.
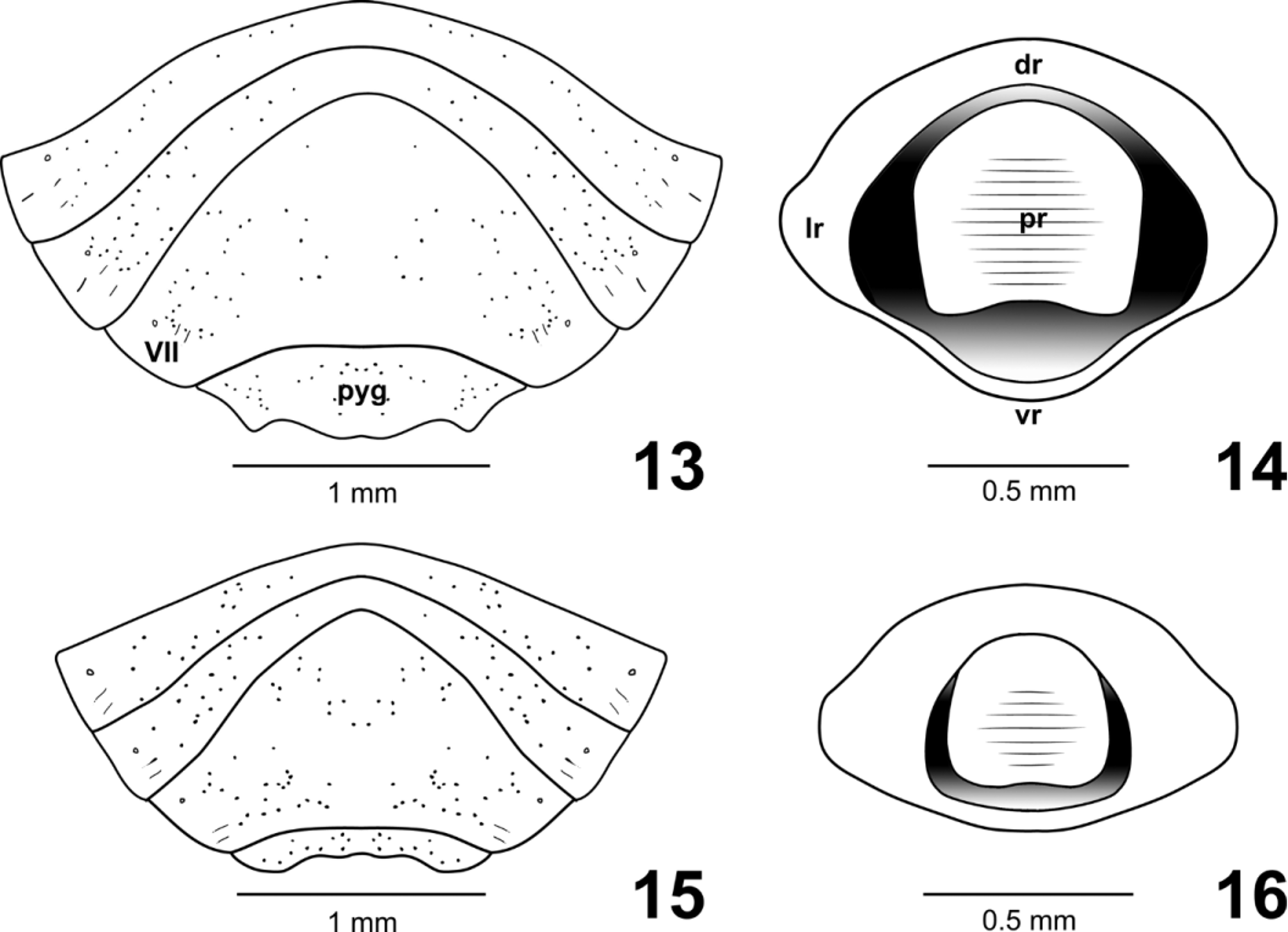
Male genitalia ( Figs. 13–16 View FIGURES 13–16 , 30, 31 View FIGURES 17–31 ). Ventral exposure of pygophore punctate. Ventral rim variable in shape. Dorsal rim flattened. Lateral rims enlarged, with dense punctures and pilosity. Proctiger wide and transversely striated, ventral half depressed. Parameres not visible externally, concealed by the proctiger.
Female genitalia ( Figs. 4 View FIGURES 1 – 4. A , 9–12 View FIGURES 5–12 , 29 View FIGURES 17–31 ). Gonocoxites 8 free from each other, triangular, sparse and finely punctate; posterior margins concave; mesial margins ventrally projected. Laterotergites 8 fused mesially, each bearing spiracle, with deep and sparse punctures. Gonocoxites 9 not visible externally; not fused mesially. Laterotergites 9 not fused, but meeting mesially, partially covering segment X, triangular, well developed, about 0.4 times the length of gonocoxites 8 at mesial margins, with deep and sparse punctures. Both tergum X and sternum X visible ventrally, the former with a median suture line. Presence of paired elongate sacs attached to base of gonocoxites 9. Ring sclerites absent. Orificium receptaculi surrounded by a narrow sclerotized groove. Ductus receptaculi connecting both to a sac-like dilation (not observed in A. atratus ) and to the pars intermedialis (with only the proximal flange developed) plus the semicircular capsula seminalis, this without projections.
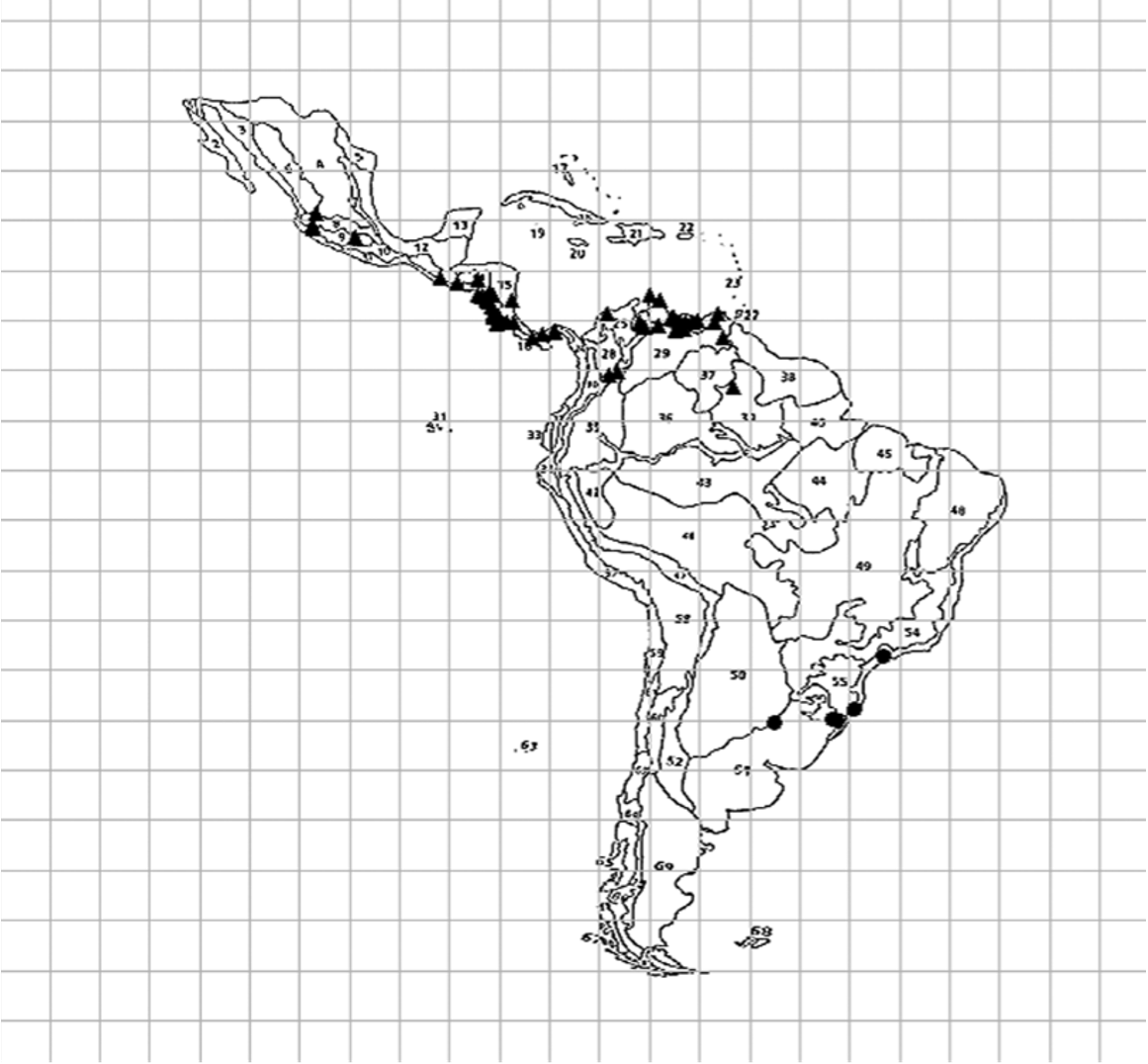
Distribution. Mexico, Guatemala, Honduras, El Salvador, Nicaragua, Aruba, Curaçao, Venezuela, Costa Rica, Colombia, Panama, and Brazil ( Fig. 32 View FIGURE 32 ).
Comments. Diagnosis. In the absence of a hypothesis of relationships among thyreocorid species, preventing the construction of a better key to the genera, specimens of Alkindus can be identified using McAtee and Malloch’s (1933) key. However, species of Alkindus are similar to, and can be confused with, those of Pericrepis Horvath, as already noted by Horvath (1919). Both genera share similarities in general color (especially with Pericrepis afer McAtee & Malloch and P. b e rg i Kormilev), head shape, position of the ocelli, elevation of the area between the costa and the exocorial vein, and female genitalia. McAtee and Malloch (1933) emphasize the sternites with bristles laterad of the trichobothria as a main character of Alkindus , while they are mostly lacking in Pericrepis. However, the presence of lateral bristles in the abdomen is not a constant character within the genus Alkindus , since they are mostly lacking in A. crassicosta . In addition to having the hind tibia dorsally sulcate, the species of Alkindus seem unique in the external morphology of male and female genitalia, allowing easy differentiation from species of Pericrepis.
External scent efferent system. The external scent efferent system of the Thyreocoridae has been poorly described. Kment and Vilímová (2010) provided SEM images of Thyreocoris scarabaeoides (Linnaeus) and line drawing of Galgupha (Gyrocnemis) impressa (Horvath) . The vestibular scar, as observed in Alkindus spp., is present in T. scarabaeoides , as well as in several other families of Pentatomoidea, ( Thaumastellidae , Cydnidae , and Plataspididae ) (Kment & Vilímová 2010). The pseudoperitreme, especially developed in A. crassicosta , has been observed in representatives of the Thyreocoridae , some Cydnidae and Plataspididae (Kment & Vilímová 2010) . A verrucose peritremal surface was described in T. scarabaeoides , differing from the smooth peritremal surface in Alkindus spp.
Leg structures. The presence of coxal combs has been regarded as a synapomorphy for the Corimelaenidae (= Thyreocoridae ) and for the Cydnidae sensu lato (including or not the Thyreocoridae ) (Dolling 1981; Grazia et al. 2008). The presence of this structure in the Thyreocoridae has been pointed out previously by some authors (e.g., McAtee & Malloch 1933; Schuh & Slater 1995; Grazia et al. 2008). More recently, Lis (2010) illustrated in SEM the coxal combs of T. scarabaeoides and Strombosoma impictum Amyot & Serville (both Thyreocorinae ). In Alkindus spp., the coxal combs seem compatible with the pattern of gutter-like setae observed for other thyreocorids and some groups of Cydnidae (Lis 2010) .
Tibial combs are present in all infraorders of terrestrial Heteroptera ; however, their presence and degree of development in the three pairs of tibiae varies greatly, as revised by Lis and Schaefer (2005). The same authors studied the tibial combs in several representatives of the Cydnidae and the Parastrachiidae , also in SEM. No Thyreocoridae species were included in their study, but they stated that the tibial combs of the fore legs are present in this family, as well as in several other pentatomoid families ( Acanthosomatidae , Dinidoridae , Pentatomidae , Plataspididae , Scutelleridae , Tessaratomidae , and Urostylididae ). Barão et al. (2013) studied the tibial comb of ten species of Thyreocoridae , in addition to Pentatomidae and Scutelleridae . They concluded that the thyreocorids differ from the pentatomids and scutellerids by the presence of the tibial comb complex (which is characteristic of the Cydnidae ), but tibial combs of thyreocorids differed only in the number of setae. According to the same authors, the number of setae in the Thyreocorinae (14–16) is usually smaller than in the Corimelaeninae (14–21), which is compatible with our observations in Alkindus spp. (19).
The foretibial apparatus is present in most groups within the superfamily (McAtee & Malloch 1928; Dolling 1981), being a unique feature of “higher” Pentatomoidea (Grazia et al. 2008). Its presence in Thyreocoridae was observed by Grazia et al. (2008) and, recently, by Barão et al. (2013). In the latter study, the foretibial apparatus of the Pentatomidae , the Scutelleridae and the Thyreocoridae differed only in the number of setae; for the Thyreocoridae , the number of setae (4–5) overlaps with the Pentatomidae (3–23). The additional data provided by the study of Alkindus spp. suggest that this number is quite conserved within the family.
Female genitalia. The female genitalia were described in only a few species of Thyreocoridae . External genitalia of several thyreocorids were illustrated by McAtee and Malloch (1933), Galgupha magna Sailer by Sailer (1941), Galgupha durionei Kormilev , Galgupha fritzi Kormilev , and Galgupha torresi Kormilev by Kormilev (1956a), Galgupha atra Amyot & Serville by Scudder (1959), Corimelaena pulicaria (Germar) by McDonald (1966), Corimelaena lateralis (Fabricius) and Corimalena obscura McPherson & Sailer by McPherson & Sailer (1978), Thyreocoris spp. by Stys & Davidová (1979), Carrabas maurus Distant, Strombosoma unipunctatum Amyot & Serville , and T. scarabaeoides by Dolling (1981), Thyreocoris pakistanensis Ahmad & Moizuddin by Ahmad & Moizuddin (1982). Female internal genitalia have been studied and illustrated only in C. pulicaria , Galgupha nitiduloides (Wolff) , Galgupha ovalis Hussey , and T. scarabaeoides (Pendergrast 1957; McDonald 1966; Stys & Davidová 1979; Pluot-Sigwalt & Lis 2008).
In almost all of those species, as well as in Alkindus spp., the laterotergites 8 are fused medially and the paired gonocoxites 8 and laterotergites 9 are free from each other (Scudder 1959; McDonald 1966). According to Dolling (1981), in T. scarabaeoides , the laterotergites 8 have a median suture line and, in S. unipunctatum , they are not fused. In Allocoris sp. (= Corimelaena ) (Corimelaeninae), the laterotergites 9 are contiguous, partially covering the segment X, as in Alkindus spp. (Grazia et al. 2008). In T. scarabaeoides , they are separate, with segment X between them (Grazia et al. 2008), as found in the other Thyreocorinae (Dolling 1981) .
The gonocoxites 9 appear to be even more variable in their degree of fusion. As observed in Alkindus spp., the gonocoxites 9 are independent from each other in the Thyreocorinae ( C. maurus , S. unipunctatum , and T. scarabaeoides ) (Dolling 1981), although Grazia et al. (2008) considered them to be joined medially by membrane in T. scarabaeoides . In Allocoris sp., they are fused, but with a distinct median fusion line (Grazia et al. 2008). In Thyreocorinae , the gonocoxites 9 are visible externally, lying between the laterotergites 9 (Dolling 1981). According to the description of McDonald (1966) for C. pulicaria and G. nitiduloides , the gonocoxites 9 are not visible externally, a condition found in all other Corimelaeninae illustrated and observed so far (including Alkindus spp.).
The receptaculum seminis (= spermathecae) appears to differ radically within the family ( McDonald 1966), but this structure could not be adequately observed in Alkindus spp. The narrow sclerotized groove surrounding the orificium receptaculi was described in G. nitiduloides ( McDonald 1966) and is similar to the observed for the Scutelleridae by Grazia et al. (2008). Among the thyreocorid species whose ectodermal female genital ducts have been studied, a dilation was present in G. nitiduloides , G. ovalis , and, in a very different shape, in T. scarabaeoides (Pendergrast 1957; McDonald 1966; Stys & Davidová 1979; Pluot-Sigwalt & Lis 2008). According to McDonald (1966), C. pulicaria would lack a dilation, but at least in one species of Corimelaena , this structure is clearly present (J. Grazia, personal communication). In A. atratus , the dilation could not be recognized, but it appears to be present as an amorphous structure in A. crassicosta . The presence of two accessory sacs has been described already in C. pulicaria , G. at r a, and G. nitiduloides , but their function is unknown (Scudder 1959; McDonald 1966). The absence of ring sclerites has been observed for other representatives of the family (Scudder 1959; McDonald 1966; Dolling 1981; Pluot-Sigwalt & Lis 2008). Those few observations support the closest relation of Alkindus with other Corimelaeninae and reinforce the need for a comprehensive comparative study on the female internal genitalia in Thyreocoridae .
Biogeographical notes. Distribution of Alkindus spp. is restricted to the Neotropical region sensu lato (Morrone 2013). This, however, is not a geological or geographically unique or homogeneous entity (Amorim 2012). Distribution records from both species are clearly disjunct. A. atratus is present in the Caribbean and Northwest components of the Neotropical region (Amorim & Pires 1996), widely distributed from Mexico to northern Brazil. On the other hand, A. crassicosta is restricted to southern and southeastern Brazil, occupying part of the Southeast Neotropical component, restricted to the Atlantic Forest, with a single record to the northwest of Pampa (Amorim & Pires 1996). This disjunct distribution is consistent with the second dichotomy of the Neotropical region sensu stricto into a north-western and a south-eastern component, as found by Morrone (2013).
Considering the classification of the Neotropical region proposed by Morrone (2013) and the biogeographical regionalization into provinces compiled by Morrone (2006), A. atratus occurs in the Mexican transition zone, the Amazonian subregion, and the South American transition zone. In the Mexican transition zone, the species occupies two provinces: the Transmexican Volcanic Belt and the Balsas Basin. In the Amazonian subregion, it occurs in all provinces of the Mesoamerican dominion (except the Mexican Gulf), in three provinces of the Northwestern South American dominion (Maracaibo, Venezuelan Coast, and Venezuelan Llanos), and in one province of the Northern Amazonian dominion (Roraima). The species has been collected in only one province of the South American transition zone (North Andean Paramo). A. crassicosta , in turn, occurs in the Chacoan subregion, more precisely in the Pampa and the Brazilian Atlantic Forest Provinces.
Lack of data from field sampling and identified museum specimens, as well as lack of hypotheses of relationships among thyreocorid genera prevent further biogeographical discussions. However, it is worth mentioning that both species of Alkindus are restricted to forest areas, since records in open formations are missing. The large gap observed in their distribution could be explained either by lack of sampling and identification or by extinction of the taxon in intervening areas. Alternatively, there may be undescribed species in areas of Cerrado and Caatinga, which should be further explored.
The availability of phylogenetic information within Thyreocoridae would be especially interesting to test the presumptive relatedness between Alkindus and Pericrepis. The latter genus is comprised of four species, distributed in Argentina (all species), Brazil (two species), and Paraguay (one species) (McAtee & Malloch 1933; Kormilev 1956b; collection data).
Key to the species of Alkindus
(modified from McAtee & Malloch, 1933)
1 Smaller (3.0– 4.8 mm); subcostal smooth area of corium broader throughout, distinctly widened in the middle of its length, the branches of exocorial vein more crowded together and subparallel ( Fig. 8 View FIGURES 5–12 ); posterior margin of abdominal sternite VII slightly projected ventrally in the female, accompanying the gonocoxites 8; gonocoxites 8 ventrally projected along entire mesial margins ( Figs. 4 View FIGURES 1 – 4. A , 11, 12 View FIGURES 5–12 ); ventral rim of pygophore broadly concave at median 3/5, scarcely covering the proctiger ( Figs. 15 View FIGURES 13–16 , 31 View FIGURES 17–31 ); distributed in southeastern and southern Brazil............................................. A. crassicosta Horvath
1’ Larger ( 4.5–5.6 mm); subcostal smooth area of corium narrower, slightly broader at base, the branches of exocorial vein divergent in middle part of their course ( Fig. 6 View FIGURES 5–12 ); posterior margin of abdominal sternite VII not ventrally projected in the female; gonocoxites 8 ventrally projected at anterior half ( Figs. 9, 10 View FIGURES 5–12 , 29 View FIGURES 17–31 ); ventral rim of pygophore convex at median third, slightly bilobate, completely covering the proctiger, with 1 + 1 acute posterior projections at lateral thirds ( Figs. 13 View FIGURES 13–16 , 30 View FIGURES 17–31 ); distributed in Mexico, Guatemala, Honduras, El Salvador, Nicaragua, Aruba, Curaçao, Venezuela, Costa Rica, Colombia, Panama, and northern Brazil........................................................................... A. atratus Distant
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Alkindus Distant, 1889
| Matesco, Viviana Cauduro & Grazia, Jocelia 2013 |
Alkindus
| Alkindus Distant, 1889: 309 |
| Lethierry & Severin 1893: 14 |
| Horvath 1919: 231 |
| McAtee & Malloch 1933: 347–348 |