Mico argentatus (Linnaeus, 1771)
|
publication ID |
https://doi.org/ 10.5281/zenodo.5730714 |
|
DOI |
https://doi.org/10.5281/zenodo.5730730 |
|
persistent identifier |
https://treatment.plazi.org/id/DF668780-FFD6-FFC1-FA3E-F7896CABEC33 |
|
treatment provided by |
Conny |
|
scientific name |
Mico argentatus |
| status |
|
3 View On .
Silvery Marmoset
French: Ouistiti argenté / German: Silberaffchen / Spanish: Titi plateado
Taxonomy. Simia argentata Linnaeus, 1771 ,
Para, Brazil. Restricted by Avila-Pires in 1969 to Cameta, left bank of lower Rio Tocantins.
This species is monotypic.
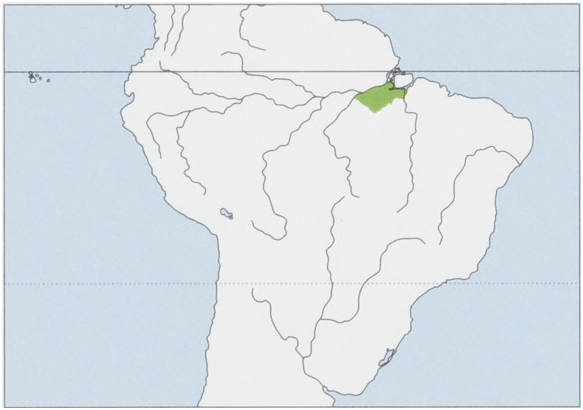
Distribution. Brazilian Amazon, S of the Rio Amazonas between the lower Rio Tocantins and Rio Tapajos, S along the right bank of the Tapajos to the Rio Cupari and left bank of the Rio Tocantins to c.4° S; between the Xingu and Tocantins,its distribution is delimited by submontane and montane forests of the Guiana Shield. View Figure
Descriptive notes. Head-body 20-22 cm,tail 26-33 cm; weight mean 349 g (males, n = 11) and 406 g (females, n = 9). The Silvery Marmosetis pale silvery-gray above and creamy-yellow below, with the two colors sharply demarcated. The tail is entirely black and not ringed, the head is creamy-white, and hands and feet are slightly darker than the body. The face and ears are naked and pinkish-red, often covered with irregular red blotches. External genitalia are red.
Habitat. Secondary and disturbed primary lowland forest. At the mouth of the Rio Tapajos (Alter do Chao), the Silvery Marmosetlives in forest patches in a sandy-soil Amazonian savanna. Groups studied in Amazonian savanna occupied home ranges that included patches of clay-soil forest, edge habitat, secondary growth, and white-sand forest (“campinarana”). Campinarana was a preferred forest type because of the high density of gum-producing trees, particularly Tapirira guianensis ( Anacardiaceae ) that also provided fruits. The Silvery Marmoset has also been studied in a forest on the east bank of the Rio Curua in young (5-year old, canopy at 6 m high) and older (agricultural plots abandoned 40 years previously, canopy 15-20 m) successional forests. A group studied at the Caxiuana National Forest, Para State, used the dense vegetation of young and older successional forest (“capoeirinha” and “capoeira”) ¢.78% of their time, edge forest 13%, igapo 6% (important during the late dry and early wet season when Symphonia globulifera nectar and Diospyros guianensis, Ebenaceae , fruits were available there), and high, mature, terra firma forest 3%. The Silvery Marmosetis sympatric with the Blackhanded Tamarin ( Saguinus niger ) between the rios Xingu and Tocantins. It is restricted to the dense lowland forests, whereas the Black-handed Tamarin occurs further south, occupying submontane and montane forests of the Guiana Shield.
Food and Feeding. Diets of the Silvery Marmoset consist of small fruits, nectar, gums, and small animal prey (arthropods and small vertebrates). A year-long study of two Silvery Marmoset groups in a 25-ha forest patch in a sandy soil savanna recorded a diet of fruits from 35 plant species, gum from eight species, and nectar of two species. Gum of Tapirira guianensis ( Anacardiaceae ) and nectar of Symphonia globulifera (Guttiferae) were important when fruits and insects were scarce in the driest months of the dry season. In a six-month study (February—July, spanning wet and dry seasons) of a group of Silvery Marmosets on the east bank of the Rio Curua, the diet comprised 63% exudates, 19% reproductive plant parts, and 18% animal prey. Fruit consumption declined and gum feeding increased in the dry season. More time was dedicated to foraging and feeding in the dry season. An earlier seven-month study of the same group found a diet of 59% exudates, 36% reproductive plant parts, and 5% animal prey; the differences were due to annual and seasonal variation. Tapirira and Symphonia were also important food resources at the Caxiuana National Forest. Species providing gum that were particularly important during the driest months (May-June) included Parkia ulei (Fabaceae) and Tapirira guianensis ( Anacardiaceae ), and those providing fruits were Myrcia atramentifera ( Myrtaceae ), Pourouma (Urticaceae) , Goupia glabra ( Goupiaceae ), and Protium (Burseraceae) . Animal prey includes Orthoptera ( Acrididae , Blattidae , Grillidae, Mantidae , Phasmidae , and Tettigoniidae ), Odonata, Hemiptera , Coleoptera , and Araneae. Silvery Marmosets follow swarms of army ants to capture small animals disturbed in thelitter.
Breeding. Births of Silvery Marmosets have been recorded in February (early wet season) and July (end of the wet season) on the east bank of the Rio Curua. A single dominant female in a group of 6-9 individuals studied at the Caxuiana National Forest gave birth to twins at six-month intervals.
Activity patterns. In a six-month study in Caxiuana National Forest, by the Rio Curua, individuals began their day between 06:20 h and 07:20 h and retired between 16:00 h and 18:05 h, being active generally for about ten hours/day. They concentrated their activities in the middle to lower canopy and understory. One study recorded that more than 90% of their activities occurred below 15 m above the ground in a forest with a canopy of 35 m. In three wet-season months, a group foraged for insects for 31% of the day, traveled 40%, fed on plant parts 9%, and rested 16%. In the dry season, they foraged more (42%), traveled slightly less (39%), spent more time feeding (13%), and spent less time resting (3%)—related undoubtedly to an energy conservation strategy, an increase in the consumption of gum, and possibly a reduced insect abundance. Foraging success (prey capture rate) was lower in the dry season. The day is typified by a predominance of traveling and feeding (first plant resources, then animal prey) in the early hours, more resting in the middle of the day, and increased feeding again later in the afternoon, prior to going to their sleeping site. Sleeping sites are typically in tall trees with vine tangles and dense foliage.
Movements, Home range and Social organization. Six groups of the Silvery Marmoset studied in forest patches in Amazonian savanna on the Rio Tapajos had 4-11 individuals. Groups had 1-2 adult females, 1-3 adult males, and various numbers of subadults, juveniles, and infants. A group studied in the Caxiuana National Forest had 6-9 individuals. At its largest, there was one adult male, two adult females, two subadults, two juveniles, and two infants. One adult female and a subadult disappeared during the study; anotherleft the group and then returned three months later. Only one female bred during the 18-month study. Home ranges of four groups were 4 ha, 5-1 ha, 9 ha, and 24 ha (a large group of 6-10 individuals). Home ranges were, to some extent, adjusted to the forest patch size, although groups traveled through corridors of bushes and trees, and even single lines of trees, to get to neighboring patches. Patches were similar in terms of availabilities of preferred fruiting trees, but space use was related to the distribution of gum sources—campinarana had the highest density of gum-producing trees. Silvery Marmosets tended to avoid edge habitat (abutting grassland), probably due to the greater risk of predation. A group of 6-9 individuals in the Caxiuana National Forest used a home range of 15-5 ha during eleven months, 24% of which overlapped with other groups. Home rangesize of the group was larger in the early and late dry season. The group traveled 630-1710 m during the day (mean 1042 m; n = 83 days), and the mean daily home range was 2-7 ha (1-4-2 ha). The group used 14 sleepingsites, with the most used sites nearer to their major food sources. The most intensively used areas were in the center of their home range and contained their main sources of gums.
Status and Conservation. CITES Appendix II. Classified as Least Concern on The [UCN Red List. The Silvery Marmoset is locally common and has a comparatively large geographic distribution. It occurs in the Tapajos National Forest (600,000 ha) to the east of the Rio Tapajos, Brazil.
Bibliography. Albernaz & Magnusson (1999), Corréa (2006), Corréa et al. (2002), Ferrari & Lopes (1990a, 1996), Goncalves et al. (2003), Hershkovitz (1977), Muniz et al. (1986), Omedes (1979, 1981, 1983), Omedes & Carroll (1980), Rylands & de Faria (1993), Rylands et al. (1993, 2009), Stevenson (1978), Stevenson & Rylands (1988), Tavares (1999), Tavares & Ferrari (2002), Veracini (1997a, 1997b, 1998, 2009).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.