Callithrix jacchus (Linnaeus, 1758)
|
publication ID |
https://doi.org/ 10.5281/zenodo.5730714 |
|
DOI |
https://doi.org/10.5281/zenodo.5730852 |
|
persistent identifier |
https://treatment.plazi.org/id/DF668780-FFDB-FFD5-FAD7-F5B269B9E414 |
|
treatment provided by |
Conny |
|
scientific name |
Callithrix jacchus |
| status |
|
23 View On .
Common Marmoset
Callithrix jacchus View in CoL
French: Ouistiti a toupets blancs / German: Weiliblischelaffchen / Spanish: Titi comun Other common names: \ White-tufted-ear Marmoset
Taxonomy. Simia jacchus Linnaeus, 1758 View in CoL ,
America. Restricted by O. Thomas in 1911 to Brazil, Pernambuco .
This species hybridizes with C. penicillata in some areas (e.g. northern Bahia State). Monotypic.
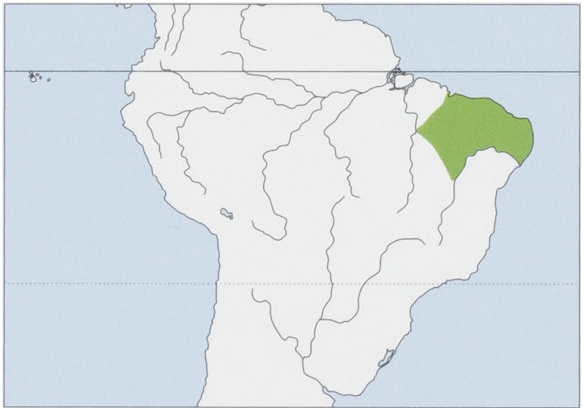
Distribution. NE Brazil (states of Maranhao, Piaui, Ceara, Rio Grande do Norte, Paraiba, Pernambuco, Alagoas, N Bahia & possibly NE Tocantins), originally extending S as far as the Rio Sao Francisco and its W bank tributary the Rio Grande (c.11° 30” S), in the NW in Maranhao and Piaui extending to the left bank of the Rio Parnaiba, probably as far as the interfluvium of the rios Itapecurt and Mearim. Introduced in numerous other regions S of the Rio Sao Francisco, Sergipe State, the N & NE of Bahia, and the more humid Atlantic Forest on the coast of the states of Espirito Santo, Rio de Janeiro, and Santa Catarina in SE Brazil. View Figure
Descriptive notes. Head-body 16-21 cm (males) and 17-20 cm (females), tail 25— 31 cm (males) and 24-30 cm (females); weight mean 317-9 g (males, n = 69) and 322 g (females, n = 86). The Common Marmoset is marbled black, gray, and yellow above, with transverse stripes on the lower back and a heavily ringed tail. Underparts are gray. The head is dark brown and dominated by white ear tufts in the form of a fan (c.3 cm in length), above and in front of the ears. There is a prominent patch of white fur on the forehead, and the crown is black. Individuals tend to be paler in drier regions and more chestnut-colored in mesic regions.
Habitat. Coastal Atlantic Forest, liana forest, semi-deciduous forest, scleromorphic cerrado woodland or cerrado savanna forest (“cerradao”), buriti ( Mauritia flexuosa) palm woodlands (“veredas”), and gallery forest in the northern parts of the central savanna (Cerrado region), and “brejo” forest (inland moist forests on the slopes of plateaus), deciduous, dry scrub forests (arboreal caatinga and “agreste” nearer the Atlantic coast), and gallery forests in the Caatinga region (semi-desert xeromorphic scrub and woodland) of north-east Brazil, and babassu palm (Orbygnia) forest along the south-eastern border of Amazonia in the states of Maranhao and Goias. The Common Marmoset can also be found in coastal scrub (“restinga”) and mangroves. Their ability to gouge tree trunks and branches to stimulate the flow of gums and latex allows them to occupy even strongly seasonal habitats that have prolonged fruit shortages. When fruit and exudate trees are abundant, the Common Marmoset can reach very high densities, especially in secondary forest, scrubland, abandoned orchards, and fruit plantations. Densities are low and group sizes are small in the most extremely seasonal habitats such as forests in the Caatinga region. Introduced populations of the Common Marmoset live in a variety of habitats, including plantations, suburban gardens, and even city parks in Rio de Janeiro. There are two major studysites of longterm field research on the Common Marmoset: since 1976, the Tapacura Ecological Station (776 ha, 110-208 m above sea level) with disturbed, secondary, semi-deciduous Atlantic Forest, 52 km from Recife, Pernamubuco, and since 1991, the experimental forestry station (180 ha) at Nisia Floresta, 45 km from Natal, Rio Grande do Norte, which has 80 ha of disturbed Atlantic Forest, reaching 20 m in height, and experimental plantations such as coconut, olives, mangos, cashew, eucalyptus, and pine.
Food and Feeding. At the Tapacura site, the marmosets depend heavily on tree and vine exudates: calumbi (a vine) Acacia paniculata, and angico Anadenanthra colubrina (both Fabaceae ), and brito Astronium fraxinifolium and cashew Anacardium occidentale (both Anacardiaceae ). The marmosets gouge holes in the bark of the trees to stimulate the flow of gum, but collect already-oozing gum (caused by damage and insect attack) from the Acacia . A. paniculata and the three tree species are found at very high densities. The 2-5-ha home range of one group contained 131 individuals of the three trees and 188 calumbi vines. The abundance and distribution of these food sources evidently vary and are important determinants of home range size and quality. In drier areas inland in southern Piaui, Common Marmosets occupy areas where gum trees are scarcer and climates are more seasonally extreme. In Sete Cidades National Park, densities of trees providing gums were as low as two per hectare, and Common Marmosets were correspondingly scarcer. The staple diet of groups of Common Marmoset in small patches of secondary forest in Joao Pessoa on the Brazilian coast was gum from Tapirira guianensis and Thyrsodium schomburgkianum (both Anacardiaceae ). All trees in the home ranges (4-5 ha) of the groups showed signs of gouging, but 1-2 trees were their principal sources of gum. Fruits eaten by Common Marmosets include Cecropia infructescences ( Urticaceae ), cashew, and caja ( Spondias sp. ) ( Anacardiaceae ), Inga edulis (Fabaceae) and jakfruit Artocarpus integrifolius ( Moraceae ), palm fruits Syagrus picrophylla ( Arecaceae ), and Cordia (Boraginaceae) . They also, very occasionally, gouge bark and eat gum of tambor ( Enterolobium contortisiliquum, Fabaceae ) and Spondias (Anacardiaceae) , but they are not important sources of food. In contrast with Tapacura, there are numerous cultivated fruit trees at Nisia Floresta that make up a large portion of the diets of Common Marmosets. One group offive individuals, studied for ten months and occupying a home range of only 2-2 ha, used two mango trees ( Mangifera indica , Anacardiaceae ), four cashew trees, and 21 copiuba trees ( Tapirira guianensis) for both gum and fruit; two seriguela trees ( Spondias purpurea) and one caja-manga tree (S. dulcis); 101 olive trees (Syzygium jambolanum, Myrtaceae ); a guava tree (Psidium guajava, Myrtaceae ); two araca trees (Psidium oligospermum); one pitanga tree (Eugenia uniflora, Myrtaceae ); and three graviola trees ( Annona muricata, Annonaceae ). Two other species provided gums: two Enterolobium contortisiliquum and seven Terminalia catappa (Combretacaeae) trees. Some of these trees fruit for a short while in the second half of the dry season (e.g. cashew and manga), but when fruits are scarce, Common Marmosets eat more gum. In the early dry season, gums can make up almost 80% of the feeding records each month. During the late dry season (December-February) and most of the wet season (March—May), fruits comprise 60%—-100% of the feeding records each month. Insect prey of the Common Marmoset include large grasshoppers, katydids ( Tettigoniidae ), crickets ( Gryllidae ), cicadas ( Hemiptera ), stick insects ( Phasmidae ), cockroaches (Blattaria), ants ( Formicidae ), and beatles ( Buprestidae ). Spiders, small lizards, eggs of a gecko (Hemidactylus mabouia), tree frogs, bird eggs and nestlings, and even an infant rodent have been recorded in their diets. Kiskadees (Pitangus), caciques (Cacicus), and tropical gnatcatchers (Polioptila plumbea) have been observed mobbing Common Marmosets, indicating that they are predators of eggs and nestlings.
Breeding. At Tapacura, Common Marmosets give birth in most months of the year (never recorded in March or August), with two birth peaks; one in October—-December (the dry season) and another in April-July (the wet season). Interbirth intervals are b—7 months. Females in larger groups have better infant survival and shorter interbirth intervals than those in smaller groups, likely because of the larger number of helpers in the group that carry infants and share food with them, reducing the burden on the breeding female. Births during a 13-month study of a group at Joao Pessoa, Paraiba State, occurred in November (middle of the dry season; September—February) and April (early wet season; March—August). Three groups of 5-15 individuals studied at Nisia Floresta also bred twice a year. Although the general rule for marmosets and tamarins is a single breeding female per social group, all three of these groups had two breeding females, believed to be mother-daughter pairs. More than one breeding female in a group is thought to be a consequence of high densities, which limit dispersal and breeding opportunities for daughters elsewhere in the area. In captivity, introduction of an unrelated male to a group can result in daughters that are not behaviorally subordinate becoming reproductively active. Only one male in each group, not related to the females, copulated with breeding females following birth, which indicates polygyny. Genetic analyses of the group members indicate that, in some cases, infants may be sired by extragroup males. When food is scarce, infant mortality can be high and especially when more than one female is breeding. This situation is accompanied by what appears to be a contest for dominance among breeding females and presumably for help from the group members in carrying infants and provisioning them with food items. Infanticide has been observed in these unstable groups. The daughter of a breeding female in one group studied in captivity assumed a dominant position when her mother was pregnant. The daughter killed her mother’s infants when they were born and those of a subsequent pregnancy. A case of polygyny in captivity, with two females giving birth within 15 days of each other, was accompanied by the mothers carrying their respective infants almost exclusively for the first two weeks. This suggested that the two females, aggressive to each other, did so to avoid infanticide. Infants are carried nearly all the time until they are about four weeks old; from then on, they are carried progressively less, with infants leaving the backs of their carriers to explore. At ten weeks old, infants are carried for ¢.20% of the time, and carrying ends at about twelve weeks. Most or all of the juvenile, subadult, and adult members of a group of Common Marmosets carry infants, but adults carry them more often and for longer periods than younger group members and are also the most active in retrieving distressed infants. Adult males engage in these behaviors more than females, and juveniles carry infants very little and for short periods. In captivity at least, reproductive success of females increases in groups with larger numbers of adult male helpers; fathers also benefit in larger groups because they carry less. When helpers are present, undue termination of nursing and unsuccessful nursing attempts by infants are less frequent, and mothers nurse the infants for longer. This indicates that helpers not only contribute directly by carrying young (reducing the burden on the female) but also promote healthier infants by beneficially influencing successful nursing by the mother. Infants are weaned at 8-10 weeks of age. Solid food is first eaten around week four, and infants are able to eat by themselves at 5-6 weeks. By weeks 12-16, infants are physically quite independent, nursing has ceased, and they eat solid food unaided. Juveniles at 4-7 months are ¢.75% of the adult weight; they play, groom, and solicit grooming and attempt to carry infants. Subadults (9-14 months) are ¢.82% of adult weight and scent-mark, show agonistic behaviors such as pilo-erection, tuft flicking, and arch walking, and help more with infant carrying if there are newborns in the group. Females reach sexual maturity at c¢.65 weeks, and males show an increase in testis size at ¢.52 weeks. Gestation is c¢.145 days and the ovulatory cycle is 28 days (range 24-30 days).
Activity patterns. A group of Common Marmosets atJoao Pessoa generally began their day c.30 minutes after sunrise at 05:15-06:10 h. They first fed on fruit or gum, followed by foraging for insects and other animal prey. As the day progressed, they rested, groomed, and played more until ¢.13:00 h, after which they resumed foraging before retiring to their sleeping sites at 16:00-17:00 h. Sleeping sites were generally in vine tangles and along branches near a tree trunk in the isolated canopiesof tall trees, near the edge of the forest and their current food sources. The daily activity budget was: foraging 24% of the day, resting 18%, eating gum 15%, grooming 15%, moving 11%, eating fruits 7%, eating insects 5%, and interacting with other groups 5%. At Tapacura,sleepingsites also tend to be near forest edge in branches in dense lianas ( Acacia paniculata; Serjania , Sapindaceae , and Gouania , Rhamnaceae ), with epiphytes in trees at heights of 4-19 m, but usually ¢.8 m above the ground. These groups begin their day at 05:00-06:05 h and retire to their sleeping sites at 16:30-17:40 h.
Movements, Home range and Social organization. Groups of Common Marmosets contain 3-13 individuals, with up to four adult females and four adult males, but generally 2-3 of each sex. Home ranges of five groups in Tapacura ranged from 2-9 ha to 6-6 ha, each overlapping 12-28%. At Nisia Floresta, two groups monitored for twelve months and a third monitored for 18 months used home ranges of 5-2 ha, 4-6 ha, and 3-9 ha. Daily movements were correspondingly small. At Tapacura, three groups traveled 528-978 m/day. At Joao Pessoa, a group studied for 13 months used an area of 5 ha. These small home ranges are undoubtedly related to the concentration and availability of gums and fruits. Trees providing fruits at Nisia Floresta are cultivated. Abundance of these fruits in the wet season and late dry season, and trees providing gum at times when fruits are scarce, allow for small home ranges, large groups, and high densities. The three groups at Nisia Floresta had 5-12 individuals, each with two reproductively active females. These grew in size, with an infant survival of 52% and despite the disappearance or emigration of six individuals and no immigration. Individuals disperse from their natal group beginning at c.17 months of age. Females disperse more often than males, and dispersal occurs more frequently during the rainy season when fruits and insects are more abundant. In five groups monitored for 36 months at Tapacura, 15 females and seven males dispersed. In ten groups monitored for ten years at the Nisia Floresta, the rate of dispersal was 0-42-3-4 ind/year, and 63-4% of the dispersing individuals were females. Reproductive competition is thought to cause dispersal. At Tapacura, densities reach 496 ind/km?, but in harsher, very seasonal habitats in the Caatinga region, for example, groups are smaller and their densities considerably lower. Predators of the Common Marmoset include the roadside hawk (Buteo magnirostris), the collared forest-falcon (Micrastur semitorquatus), and the Jaguarundi (Puma yagouaroundz).
Status and Conservation. CITES Appendix II. Classified as Least Concern on The [UCN Red List. The Common Marmoset was once extensively exploited for the exotic pet trade and biomedical research. Although widespread and common in many localities, and even replacing other species of Callithrix where it has been introduced, populations of Common Marmosets are depleted through habitat destruction in extensive parts of their original geographic distribution. The following protected area are within their geographical range: Sete Cidades National Park, Serra da Capivara National Park, Ubajara National Park, Serra Negra Biological Reserve, Saltinho Biological Reserve, Pedra Talhada Biological Reserve, Guariba Biological Reserve, Mamanguape Ecological Station, Serido Ecological Station, Itabaiana Ecological Station (possible hybrids with Black-tufted-ear Marmoset, C. penicillata ), Urucui-Una Ecological Station, Aiuaba Ecological Station, Foz do Sao Francisco Ecological Station, Raso da Catarina Ecological Reserve (possible hybrids with the Black-tufted-ear Marmoset), Ponta do Cabo Branco State Park, Guaramiranga State Park, Dunas Costeiras State Park, Buraquinho State Biological Reserve, and Tapacura State Ecological Station. Tijuca National Park, in Rio de Janeiro State, contains an introduced population.
Bibliography. Abbott (1978, 1984a), Abbott & Hearn (1978), Abbott et al. (1993), Albuquerque & Arruda (1997), Alonso (1986), Alonso & Langguth (1989), Alonso et al. (1987), Anzenberger (1985), Araujo et al. (2000), Arruda et al. (1986a, 1986b), Barrett et al. (1990, 1993), Box (1975a, 1975b, 1977a, 1977b), Coimbra-Filho (1971, 1972, 1984, 1990), Coimbra-Filho & Camara (1996), Coimbra-Filho & Mittermeier (1976, 1977a), Coimbra-Filho et al. (1980), Digby (1995, 1999), Digby & Barreto (1993, 1996, 1998), Digby & Ferrari (1994), Digby & Saltzman (2009), Digby et al. (1996), Epple (1970), Erkert (1989), Evans & Hodges (1984), Evans & Poole (1983, 1984), Faulkes et al. (2003), Garber et al. (2009), Harding et al. (1982), Hearn (1978, 1982), Hershkovitz (1977), Hubrecht (1984, 1985), Kendrick & Dixson (1983), Koenig (1995), Koenig & Rothe (1991a, 1991b), Lazaro-Perea (2001), Lazaro-Perea, de Castro et al. (2000), Lazaro-Perea, Snowdon & Arruda (1999), Maier et al. (1982), Melo et al. (2003), de Menezes (2004), Mills et al. (2004), Monteiro da Cruz (1998), Monteiro da Cruz & Scanlon (1997), Nagamachi, Pieczarka & Barros (1992), Nagamachi, Pieczarka, Schwarz et al. (1994), Neusser et al. (2005), Nievergelt et al. (2000), Norcross & Newman (1993), de Oliveira et al. (1999), Olmos (1993), Peters & Guerra (1998), Pontes & Monteiro da Cruz (1995), Roda & Pontes (1998), Moura (2007), Rothe (1975), Rothe & Koenig (1991), Rothe, Darms et al. (1993), Rothe, Koenig & Darms (1993), Ruiz-Miranda, Affonso, Martins & Beck (2000), Ruiz-Miranda, Affonso, Morais et al. (2006), Rylands, Coimbra-Filho & Mittermeier (1993, 2009), Rylands, da Fonseca et al. (1996), Rylands & de Faria (1993), Saltzman et al. (2004), Santee & Arruda (1994), dos Santos & Monteiro da Cruz (1997), Scanlon, Chalmers & Monteiro da Cruz (1988, 1989), Scanlon, Monteiro da Cruz & Rylands (1991), Silva, G.S. & Monteiro da Cruz (1993), Silva, J.S. (1999), de Sousa, Albuquerque et al. (2009), de Sousa, Peregrino et al. (1999), de Sousa, Silva et al. (1999), Stevenson (1976a, 1976b, 1976¢, 1978b), Stevenson & Poole (1976), Stevenson & Sutcliffe (1978), Stevenson & Rylands (1988), Tardif (1994, 1997), Tardif & Garber (1994), Tardif & Jaquish (1997), Tardif, Carson & Gangaware (1986), Tardif, Harrison & Simek (1993), Tardif, Power et al. (2002), Tardif, Santos et al. (2002), Wohnus & Benirschke (1966), Ximenes & de Sousa (1996), Yamamoto (1993, 2005), Yamamoto & Box (1997), Yamamoto, Arruda et al. (2009), Yamamoto, Box et al. (1996).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Callithrix jacchus
| Russell A. Mittermeier, Anthony B. Rylands & Don E. Wilson 2013 |
Simia jacchus
| Linnaeus 1758 |