Arostrilepis rauschorum, Makarikov, Arseny A., Galbreath, Kurt E. & Hoberg, Eric P., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3608.6.1 |
|
publication LSID |
lsid:zoobank.org:pub:32AAC94B-5793-4D51-8DCC-AF2D8AD5BCBD |
|
DOI |
https://doi.org/10.5281/zenodo.6147206 |
|
persistent identifier |
https://treatment.plazi.org/id/E07D87D9-FFED-651E-68BA-F9DABC998260 |
|
treatment provided by |
Plazi |
|
scientific name |
Arostrilepis rauschorum |
| status |
sp. nov. |
Arostrilepis rauschorum sp. n.
( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 )
Type host: Microtus oeconomus Pallas ( Rodentia : Cricetidae : Arvicolinae ).
Other hosts: Microtus longicaudus (Merriam) , M. pennsylvanicus (Ord) , M. miurus Osgood and M. xanthognathus (Leach) .
Type locality: Adjacent toToolik Lake, Brooks Range, Alaska, near US Department of Energy research site (ca., 68o38’N, 149o36’W).
Other localities: North-central interior, Alaska, near Bonanza Creek Research Station (62o42’N, 148o16’W); Gates of Arctic National Preserve, Brooks Range, Alaska, SE side of Walker Lake (67o06’N, 154o16’W); Yukon Charley Rivers National Preserve, Alaska, across Yukon River from Glenn Creek Cabin (65o30’N, 142o03’W); Kobuk Valley National Park, Alaska, Baird Mountains, Salmon River (67o36’11”N, 159o47’20”W); and Pattee Canyon, Missoula Co., Montana (46o48’N, 113o57’W, and 46o49’N, 113o58’W).
Type material: Holotype MSB Para 1208 (field number FIN 4072484) from type host and locality by H. Henttonen and G. Batzli, 24 July 1984. Paratypes MSB1360, 1362 (AF 37462 C1/ cyt- b sequence; 37462 C3) ex M. pennsylvanicus , by H. Henttonen at Bonanza Creek, 1 August 2000; MSB 1204 (FIN 2070784) from type host species and locality by H. Henttonen and G. Batzli, 7 July 1984; MSB 1211 (FIN 5220884) from type host species and locality by H. Henttonen and G. Batzli, 22 August 1984; MSB 1205, 1206 (FIN 3120884-1, 2 slides; 3120884- 2) from type host species and locality by H. Henttonen and G. Batzli, 12 August 1984; MSB 1363 (AF 42531/ cytb sequence) from type host and locality by H. Henttonen, 8 August 2000; MSB 1365 (AF 42657) ex M. oeconomus by H. Henttonen et al., near Toolik Lake, 8 August 2000; MSB 1215 (JMK 02-04) ex M. longicaudus , by J.M. Kinsella at Pattee Canyon, Montana (46o48’N, 113o57’W), 8 October 2002; MSB 1369 (AF 49499/ cyt- b sequence) ex M. longicaudus by H. Henttonen et al., from Yukon Charley, 8 August 2001; MSB 1370, 1371 (AF 59099 C3, 2 slides; 59099 C4, 2 slides) ex M. xanthognathus by A. M. Runck et al., from Gates of the Arctic National Preserve, 6 August 2002; and MSB 1216 (JMK 2009, 2 slides) ex M. pennsylvanicus , by J.M. Kinsella at Pattee Canyon, Montana (46o49’N, 113o58’W), 20 August 2009. See Appendix 1 for listing of additional identified voucher specimens.
Symbiotype: Host specimen not deposited in a museum archive.
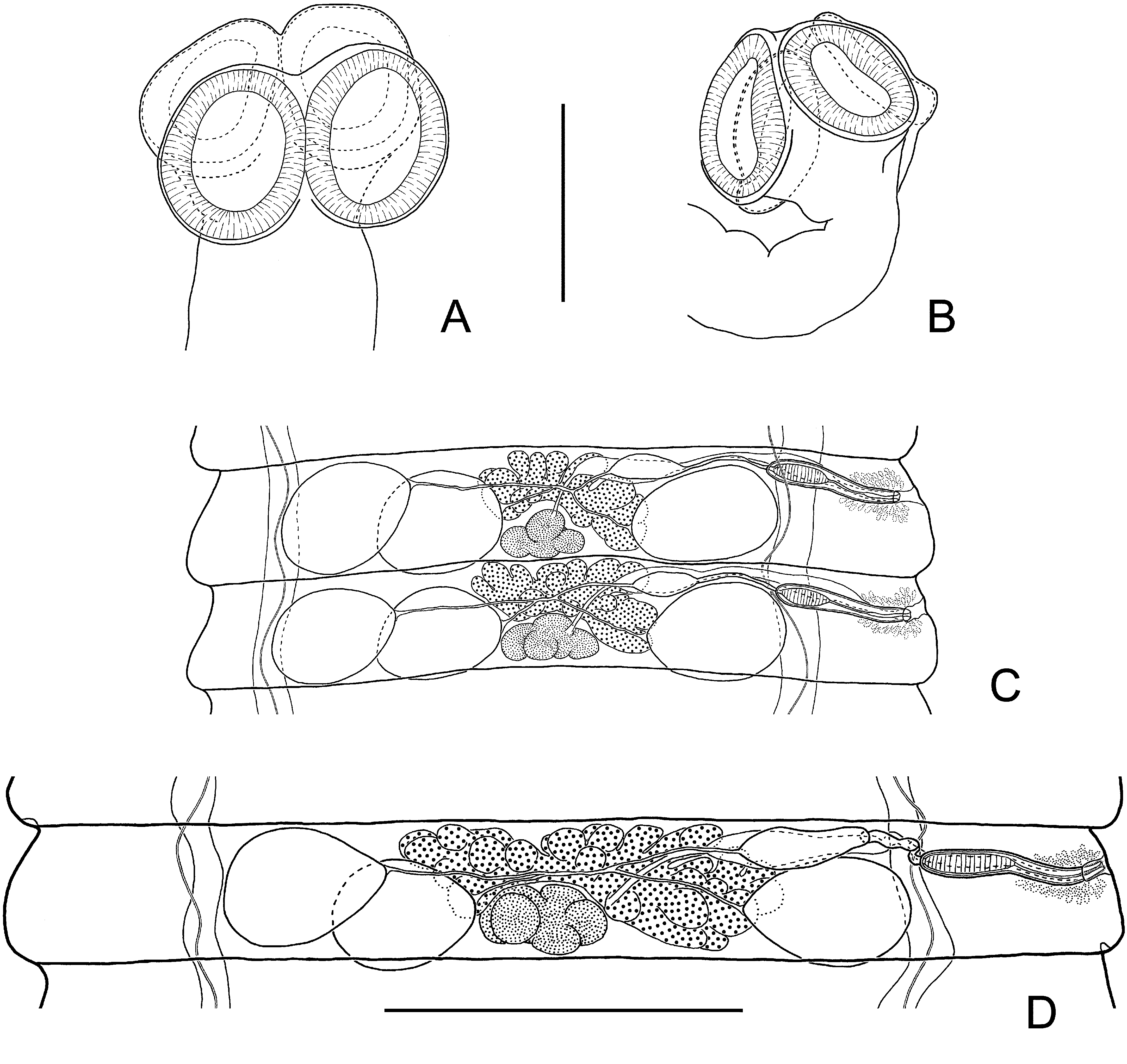
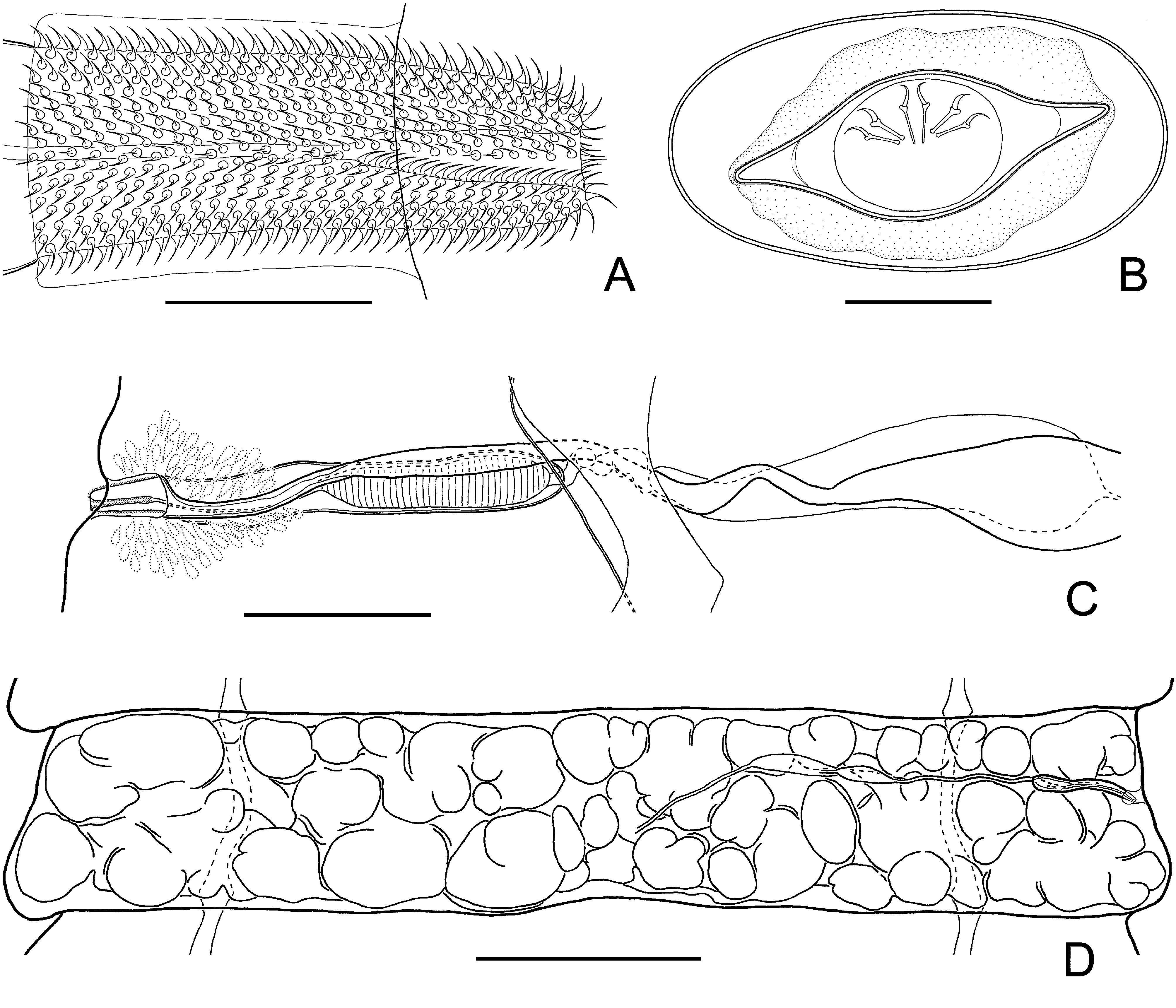
Description: Based on 13 specimens. Fully developed strobila 120–185 mm long, with maximum width at pregravid or gravid proglottides, 2.4–3.8 mm. Strobila flat, consisting of 750–950 craspedote proglottides. Scolex slightly compressed dorso-ventrally, 240–300 (266, n = 7) wide, clearly wider than neck. Suckers unarmed, ovoid in surface view, relatively small, 130–190 × 120–155 (166 × 137, n = 15), with thin walls ( Fig. 6 View FIGURE 6 A, B). Rhynchus and rostellar apparatus absent. Neck relatively long and narrow, 125–190 (152, n = 10) wide.
Two pairs of osmoregulatory canals, without transverse anastomoses. Dorsal osmoregulatory canals thin, 2–3.5 (2.6, n = 10) wide, situated predominantly in same sagittal plane as ventral canals. Ventral osmoregulatory canals 30–58 (42, n = 10) wide. Position of dorsal osmoregulatory canals not always constant; loops may be situated laterally to ventral canals. Genital pores unilateral, dextral. Genital ducts usually pass dorsally to longitudinal osmoregulatory canals, position of genital ducts between osmoregulatory canals within same strobila appears rarely (for no more than 10% proglottides) ( Fig. 6 View FIGURE 6 C, D). Development of proglottides gradual, protandrous. Strobilar part containing juvenile proglottides without external segmentation; proglottides become externally distinct at level of premature part of strobila.
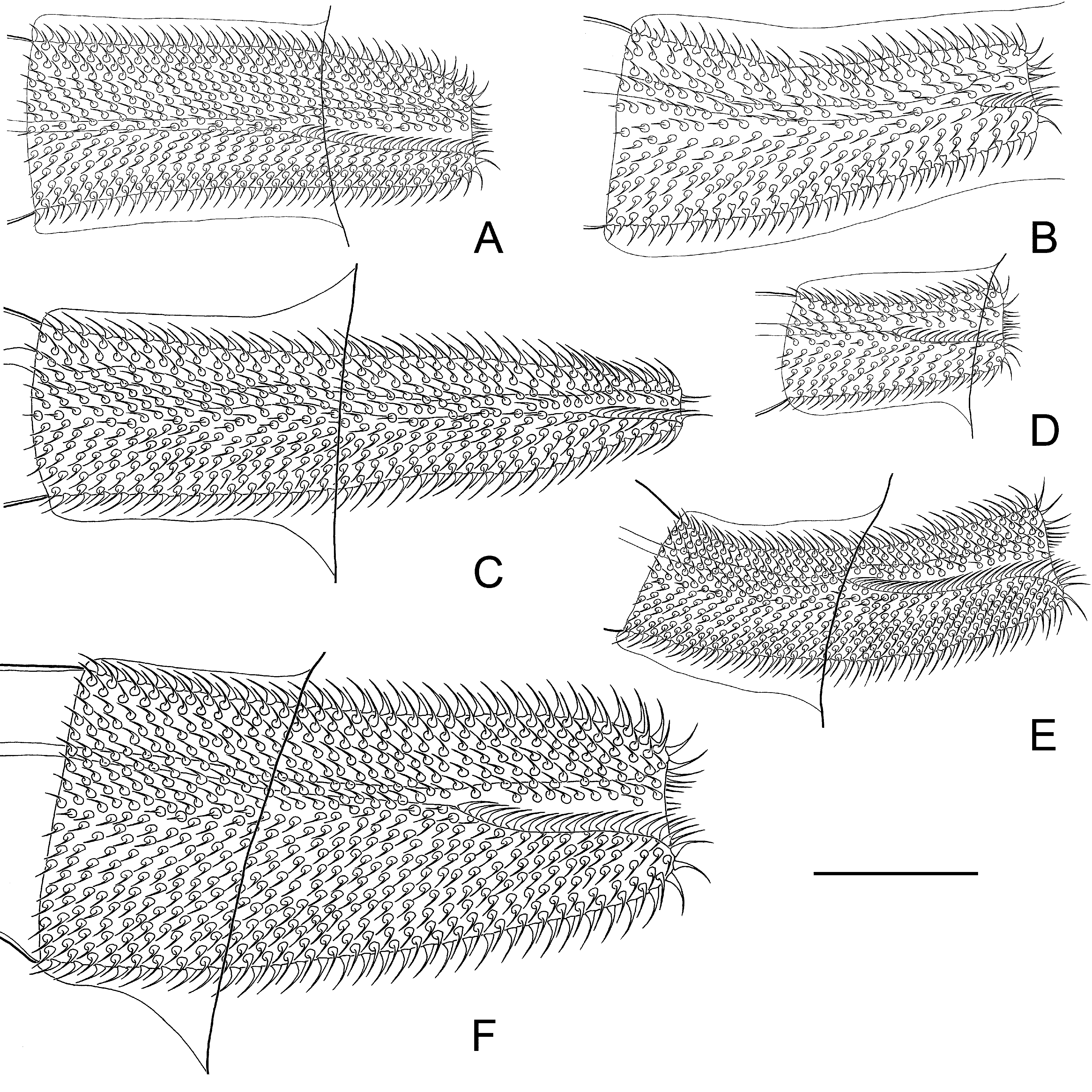
Mature proglottides 170–230 × 1330–1700 (202 × 1485, n = 16), transversely elongate, trapeziform ( Fig. 6 View FIGURE 6 C, D). Testes relatively large, usually three in number, almost of equal size, 130–252 × 110–194 (217 × 164, n = 35), oval or pear-shaped, commonly situated in one row; poral testis separated from two antiporal testes by female gonads. Arrangement of testes may vary (triangle or triangle with flat angle). Cirrus-sac relatively large, 210–242 × 31–42 (225 × 37, n = 23), with well-developed external muscular layers. Antiporal part of cirrus-sac commonly not reaching or rarely overlapping ventral longitudinal canal ( Figs. 6 View FIGURE 6 D, 7C). Genital atrium simple, cup-shaped, deep, opens laterally about middle or slightly anterior of lateral proglottis margin. Cirrus 77–92 (83, n = 18) long, conical, with relatively wide basal region, 18–23 (19, n = 18) in diameter, and narrow distal region, 12–15 (13, n = 18) in diameter; armed along entire length with relatively large (up to 3.5–4 long) rosethorn-shaped spines ( Fig. 7 View FIGURE 7 A). Internal seminal vesicle with circular musculature, ovoid, 87–122 × 25–38 (102 × 30, n = 23), shorter than half of cirrus-sac length ( Figs. 6 View FIGURE 6 D, 7C). External seminal vesicle transversely elongate, 165–270 × 55–87 (218 × 70, n = 18), clearly outlined from vas deferens, with size approximately equal to seminal receptacle.
Ovary 455–585 (537, n = 25) wide, median, fan-shaped, irregularly lobed, ventral to male genital organs, occupying substantial part of median field, slightly overlapping testes ( Fig. 6 View FIGURE 6 D). Vitellarium 75–123 × 145–240 (96 × 193, n = 25), postovarian, median, scarcely lobed. Vagina tubular, clearly distinct from seminal receptacle; ventral to cirrus-sac. Distal part of vagina 96–120 × 8–18 (107 × 13, n = 12), thick-walled, covered externally by dense layer of intensely stained cells; poral part of vagina infundibular ( Fig. 7 View FIGURE 7 C). Conductive part of vagina 170–230 × 9–30 (210 × 19, n = 10), thin-walled, vastly varying in diameter depending on degree of distention with sperm. Seminal receptacle relatively small, transversely elongate, 165–280 × 50–92 (220 × 68, n = 18).
Uterus appears as complex of fine-walled anastomosing tubes of varying length and diameter, positioned ventrally to other organs. With development of proglottides, tubular structures increase in width and uterus becomes labyrinthine. Testes remain in postmature and pregravid proglottides; cirrus-sac and vagina persist in gravid proglottides. Gravid proglottides transversely elongate, 300–500 × 1750–3800 (373 × 2600, n = 16). Fully developed uterus labyrinthine, occupying entire median field and extending bilaterally beyond longitudinal osmoregulatory canals ( Fig. 7 View FIGURE 7 D). Uterus contains numerous (up to 2100) eggs. Eggs 22–35 × 50–68, elliptical, with thin outer coat (up to 0.5); oncosphere 11–17 × 15–22 ( Fig. 7 View FIGURE 7 B). Embryophore fusiform, 14–20 × 38–47, with straight polar processes. Embryonic hooks small, 7.5–8.3 long.
Etymology: Arostrilepis rauschorum sp. n. is named in honor of Robert L. Rausch and Virginia R. Rausch in recognition of their seminal and critical studies of parasites of arvicoline rodents, rodent systematics and biogeography at high latitudes of the Holarctic, and insights about the historical development of the Beringian fauna. Further, with the passing of Robert Rausch on 6 October 2012, this species and our recent studies across Beringia are dedicated to his memory and the legacy established by an extraordinary pioneer of parasitology and mammalogy in the north.
Remarks: Arostrilepis rauschorum sp. n. is distinguished from congeners by the length and shape of the cirrus (Table 2). In A. rauschorum the cirrus is longer relative to those in A. beringiensis , A. tenuicirrosa , A. mariettavogeae , A. schilleri and A. gulyaevi , but smaller in comparison to A. macrocirrosa and A. cooki ( Figs. 11 View FIGURE 11 , 12 View FIGURE 12 ). The cirrus is armed with relatively large rosethorn-shaped spines and has a conical form; these features distinguish A. rauschorum from A. beringiensis , A. intermedia , A. janickii and A. schilleri (cylindrical cirrus), A.
microtis and A. gulyaevi (cirrus with wide conical basal region and a cylindrical distal region) and A. tenuicirrosa (cirrus armed with relatively small needle-shaped spines and having a conical basal region and a very narrow cylindrical distal region). Arostrilepis rauschorum is characterized by a relatively wide strobila and ovary and a large seminal receptacle. The cirrus-sac is shorter than in A. horrida , but longer than in A. beringiensis , A. intermedia , A. janickii , A. mariettavogeae and A. schilleri . The egg and oncosphere are large relative to those in A. janickii , A. mariettavogeae and A. schilleri (see Table 2). The proximal end of the cirrus-sac in hermaphroditic mature proglottides commonly does not reach the ventral longitudinal canal or rarely overlaps it. Specimens of A. rauschorum are distinguished from A. horrida , A. macrocirrosa , A. tenuicirrosa , A. intermedia , A. schilleri , A. gulyaevi and A. cooki as its testes form one row; in the latter species, the testes are arranged in a triangle. Furthermore, the gravid proglottides are transversely elongate and the polar processes of the embryophore are straight in A. rauschorum . This species is a specific parasite of voles of the genus Microtus from North America.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.