Salmoneus shojaei, Ashrafi & Anker & Ďuriš, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5165.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:894BAEDF-3CE4-4564-8DC9-547D12B42919 |
|
DOI |
https://doi.org/10.5281/zenodo.6839760 |
|
persistent identifier |
https://treatment.plazi.org/id/E3370029-5D2E-FFE8-FF33-FEBF9E13FC83 |
|
treatment provided by |
Plazi |
|
scientific name |
Salmoneus shojaei |
| status |
sp. nov. |
Salmoneus shojaei sp. nov.
( Figs. 1 4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Type material. Iran. Holotype: ovig. specimen (cl 5.30 mm), MNHN-IU-2014-1257, Persian Gulf , Khamir Port, 26°58’45.37”N, 55°39’14.36”E, mangrove forest, 25 May 2019, leg. H. Ashrafi [fcn IR19-110] GoogleMaps . Paratypes: 1 ovig. specimen (cl 5.63 mm), UF 60587 , same collection data as for holotype [fcn IR19-110] GoogleMaps ; 1 non-ovig. specimen (cl 5.73 mm), MNHN-IU-2014-1259, same collection data as for holotype [fcn IR-19-111] GoogleMaps ; 1 non-ovig. specimen (cl 5.40 mm), ZUTC 6920 , Persian Gulf , northern coast of Qeshm Island, 26°48’8.70”N, 55°43’5.30”E, mangrove forest, 17 May 2017, leg. M. Shojaei [fcn IR17-103] GoogleMaps ; 2 non-ovigerous specimens (cl 4.79 mm, second specimen’s carapace severely damaged), MNHN-IU-2014-1260 GoogleMaps ; same data as for previous specimen [fcn IR17-104] GoogleMaps ; 1 non-ovig. specimen (cl 4.5 mm), MNHN-IU-2014-1261, Persian Gulf, Qeshm Island, Laft Port, 26°52’49.02”N, 55°46’13.77”E, mangrove forest, 15 Dec. 2007, leg. R. Naderloo [fcn IR07-101] GoogleMaps ; 1 ovig. specimen (cl 4.75 mm), MNHN-IU-2014- 1263, Persian Gulf, Qeshm Island, no further details, 26 Oct. 2009, leg. R. Naderloo [fcn IR09-101] GoogleMaps ; 1 non-ovig. specimen (cl 5.13 mm), ZUTC 6921 , Gulf of Oman, Tiab Port, 27°5’10.16”N, 56°49’52.44”E, mangrove forest, 14 Apr. 2009, leg. R. Naderloo [fcn IR09-102] GoogleMaps ; 2 ovig. specimens (cl 5.50 and 5.83 mm), FLMNH UF 60586 GoogleMaps ; Gulf of Oman, Guwatr Bay, 25°12’1.06”N, 61°32’33.75”E, 25 Apr. 2019, leg. H. Ashrafi [fcn IR19-108] GoogleMaps .
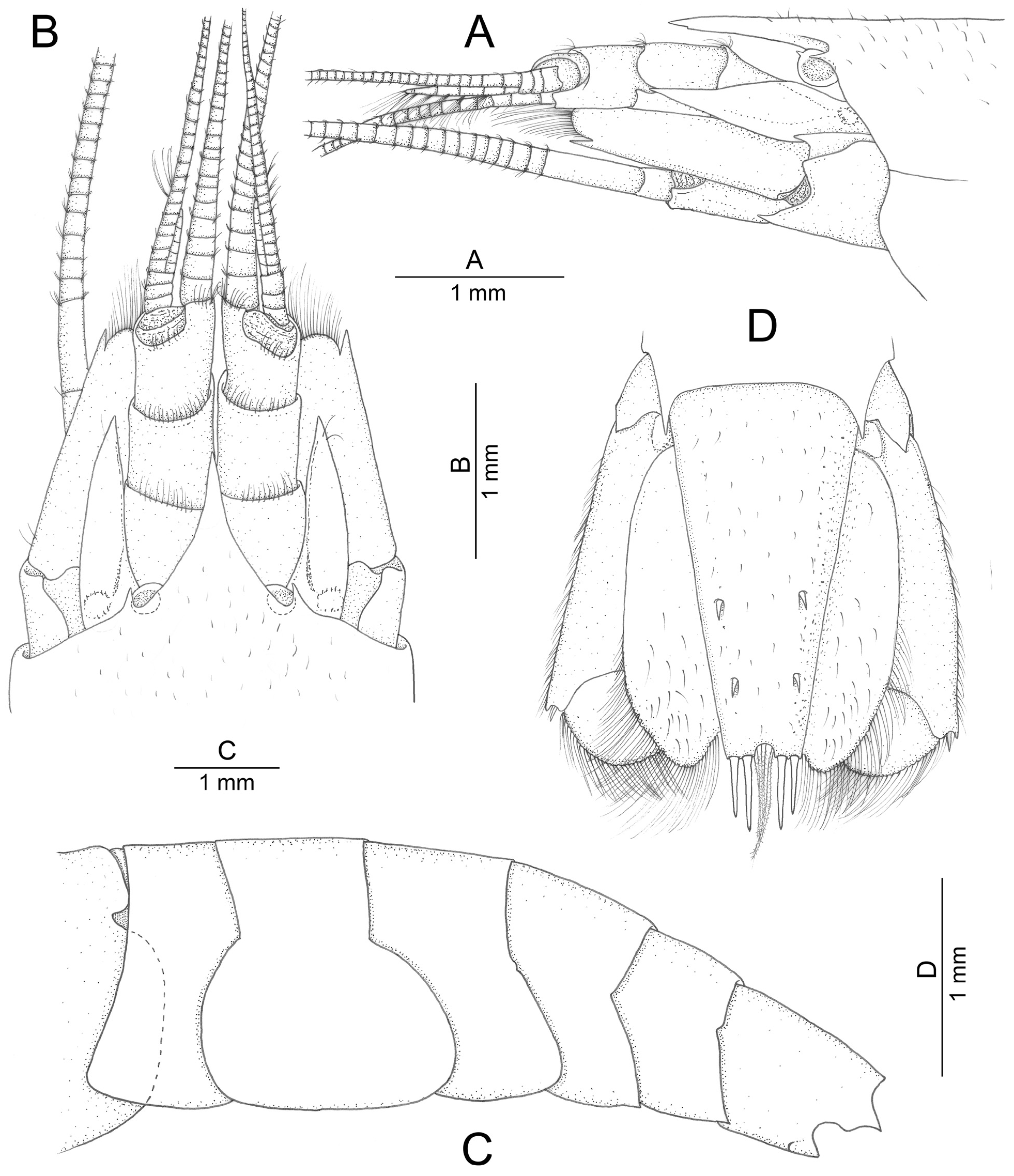
Description of holotype. Small-sized alpheid shrimp. Carapace ( Fig. 1A, B View FIGURE 1 ) smooth, covered by short setae mainly dorsally, almost completely concealing eyes dorsally (except for small most-anterior portion), only partly concealing eyes laterally; antero-lateral suture present; pterygostomial angle broadly rounded; cardiac notch deep. Rostrum ( Fig. 1A, B View FIGURE 1 ) triangular, slightly longer than broad, with subacute tip, latter reaching to middle of second article of antennular peduncle; lateral margins slightly concave; rostral carina not distinct; ventral margin with minute subdistal tooth. Orbital teeth ( Fig. 1A, B View FIGURE 1 ) small, about 0.1 of rostrum length, narrow, acute, not extending beyond eyes. Eyestalks with corneas somewhat reduced; anterodorsal margin bearing small triangular tooth ( Fig. 1A View FIGURE 1 ).
Pleon ( Fig. 1C View FIGURE 1 ) with pleura of first to third pleonite rounded antero- and posteroventrally; fourth to sixth pleura acutely produced posteroventrally; sixth pleonite with subtriangular projection flanking telson and incomplete oblique suture at posteroventral angle.
Telson ( Fig. 1D View FIGURE 1 ) slender, subrectangular, tapering distally, more than five times as long as distal width; dorsal surface with two pairs of small spiniform setae situated at about 0.6 and 0.8 telson length, respectively; posterior margin with two pairs of slender spiniform setae, mesial slightly longer than lateral, and deep U-shaped median notch, latter with several long plumose setae.
Antennular peduncle ( Fig. 1A, B View FIGURE 1 ) relatively stout; first article as long as wide; stylocerite relatively broad, with subacute tip, slightly overreaching middle of second article; second article slightly longer than wide, with deep notch laterally, slightly longer than first and third articles; lateral antennular flagellum with two rami fused basally, fused portion composed of three subdivisions, shorter ramus composed of six poorly demarcated subdivisions bearing eight or so groups of aesthetascs.
Antenna ( Fig. 1A, B View FIGURE 1 ) with basicerite stout, armed with sharp distoventral tooth; dorsal margin with blunt projection; scaphocerite not overreaching antennular peduncle, its distolateral tooth reaching anterior margin of blade, latter about three times as long as broad; carpocerite short, reaching half-length of scaphocerite, failing to reach end of second article of antennular peduncle; flagellum not particularly thickened, moderately slender.
Third maxilliped ( Fig. 3A View FIGURE 3 ) slender; coxa with strap-like epipod and prominent rounded lateral plate; antepenultimate article more than 2.5 times as long as penultimate article, with short scattered setae; penultimate article four times as long as wide, with few long setae distally; ultimate article tapering distally, with one apical spiniform seta, some long setae subdistally and multiple transverse rows of short serrulate setae ventrally and ventromesially; exopod well developed, subequal to antepenultimate article in length; arthrobranch well developed, but not particularly enlarged.
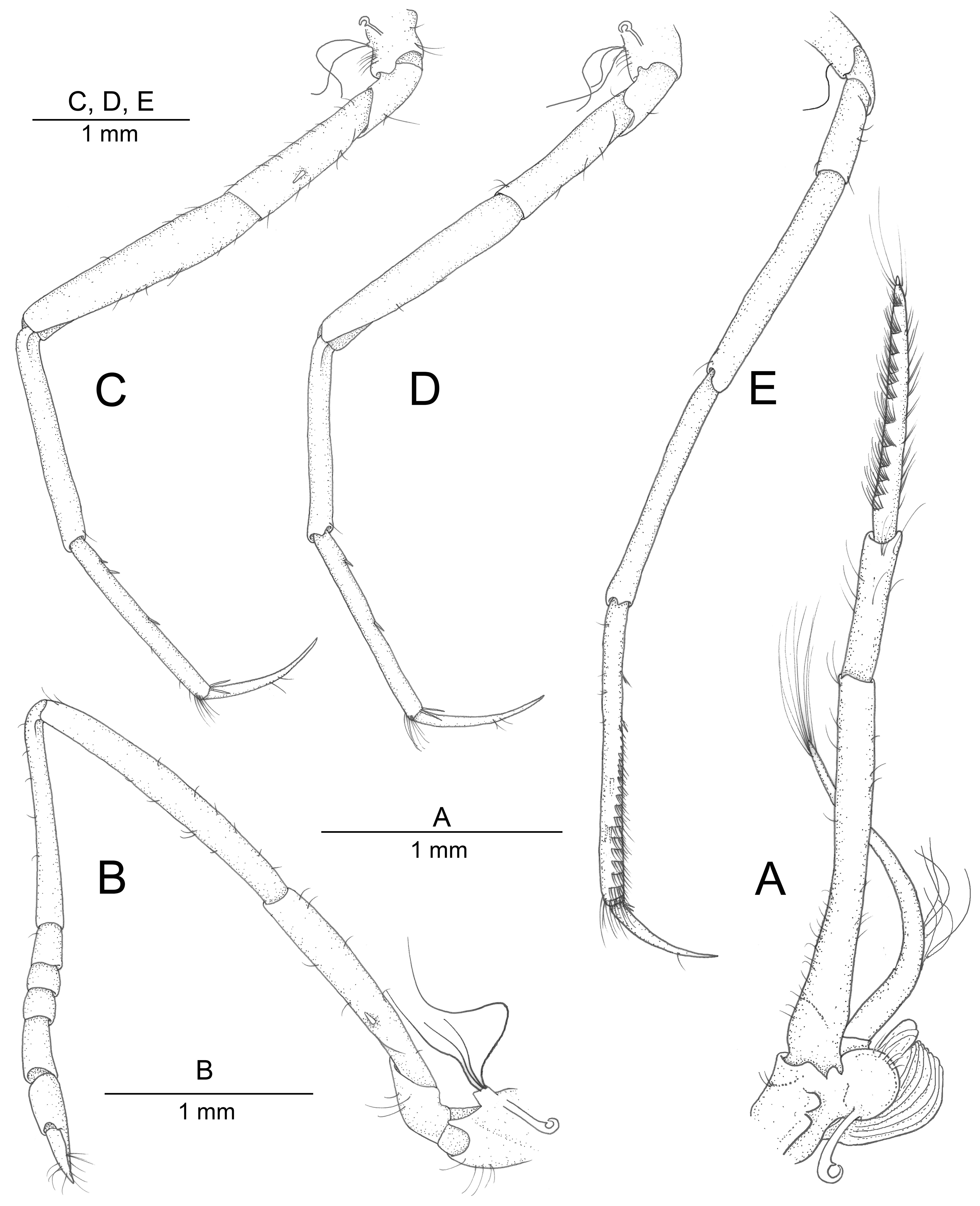
First pereiopods = chelipeds ( Fig. 2A–E View FIGURE 2 ) very different in shape, asymmetrical in size. Major cheliped ( Fig. 2A–C View FIGURE 2 ) robust, carried flexed under body at rest; ischium slender, unarmed ventrally, about three times as long as wide; merus long, slender, about 0.6 of carapace length and three times as long as distal height, slightly concave ventrally, feebly widening towards distal margin; carpus cup-shaped, short, as long as distal width; chela enlarged, swollen, as long as merus and ischium combined, with smooth surfaces; palm robust, about twice as long as maximal width, about 0.9 of merus length, distinctly longer than fingers, somewhat flattened distodorsally, with shallow depression extending from deep ventroproximal groove and very faint ripples on ventrolateral surface; fingers not gaping when closed, somewhat twisted mesially; fingertips hook-shaped, crossing subapically; cutting edges with 15–20 subequal, small, evenly-spaced teeth. Minor cheliped ( Fig. 2D, E View FIGURE 2 ) much smaller and weaker than major cheliped, slender; ischium elongate, more than three times as long as wide at its mid-length, with small spiniform seta on ventrolateral margin; merus slightly longer than ischium, somewhat curved, not swollen, about five times as long as maximal width; carpus slender, subcylindrical, subequal to merus in length, expanding distally; chela about 0.7 of carpus length; palm slightly longer than fingers; fingers simple, with unarmed cutting edges.
Second pereiopod ( Fig. 3B View FIGURE 3 ) moderately slender; basis short, stout; ischium elongate, about six times as long as wide, with one spiniform seta on ventrolateral surface, at about 0.4 of article length; merus long, about 1.5 times as long as ischium; carpus slender, with five divisions, first (proximal) longer than combined length of remaining ones; approximate length ratio of carpal subdivisions equal to 14: 3: 2: 2: 4.
Third to fifth pereiopods ( Fig. 3C–E View FIGURE 3 ) slender. Third pereiopod ( Fig. 3C View FIGURE 3 ) with ischium elongate, about four times as long as wide, with one spiniform seta on ventrolateral surface; merus about 1.3 times as long as ischium, about 4.7 times as long as maximal width; carpus distinctly slenderer and slightly shorter than merus; propodus subequal to carpus; ventral margin bearing three short spiniform setae, two proximally and one at mid-length, and one pair of longer spiniform setae distally, near dactylar base; dactylus slender, gently curved, simple, more than 10 times as long as basal width, about 0.7 of propodus length. Fourth pereiopod ( Fig. 3D View FIGURE 3 ) generally similar to third pereiopod, slightly slenderer; ischium about four times as long as wide, unarmed; merus more than five times as long as maximal width; carpus slenderer, about 0.9 as long as merus; propodus subequal to carpus; ventral margin with two widely spaced, short spiniform setae, both in its proximal half, and one pair of longer spiniform setae distally, near dactylar base; dactylus as in third pereiopod, about 0.6 as long as propodus. Fifth pereiopod ( Fig. 3E View FIGURE 3 ) slenderer than fourth pereiopod; ischium short, about three times as long as wide, unarmed; merus about seven times as long as maximal width; carpus about as long as merus; propodus about 1.2 times as long as carpus; ventral margin with three or four widely spaced, short spiniform setae (two distal ones not visible in lateral view), and one or two longer spiniform setae near dactylar base; propodal cleaning brush well developed, composed of 16–17 transverse rows of microserrulate setae extending from almost mid-length of propodus to its distal end; dactylus slender, simple, slightly shorter than half of propodus, otherwise similar to that of third and fourth pereiopods.
Uropod ( Fig. 1D View FIGURE 1 ) with lateral lobe of protopod produced into sharp or subacute tooth; exopod moderately broad, ovate, with small distolateral tooth adjacent to short spiniform seta; diaeresis sinuous, with blunt lateral projection; endopod as long as exopod, narrower, without specific features.
Variation. The subdistal tooth on the ventral margin of the rostrum varies from small to minute; in two paratype specimens (MNHN-IU-2014-1259 and MNHN-IU-2014-1263), there is no trace of this tooth. The number of spiniform setae on the third pereiopod ischium varies from one to two, whereas the accessory spiniform setae on the distal mesial surface of the fifth pereiopod propodus are present only in the holotype.
Colour pattern. Body translucent with red chromatophores present on anterodorsal and anterolateral portions of carapace, especially posterior to rostrum and near pterygostomial area, on antennular peduncles, as well as on pleon and telson, on pleon forming faint diffused transverse bands, with most chromatophores concentrated closer to posterior margin of each pleonite; eggs pale green ( Fig. 4 View FIGURE 4 ).
GenBank accession numbers. See Table 1.
Etymology. The new species is named after Dr. Mehdi Shojaei (Tarbiat Modarres University, Iran), who is actively working on biodiversity and conservation of mangrove forests in Iran, and who also generously sponsored HA’s mangrove field studies. Dr. M. Shojaei’s last name is used as a noun in apposition.
Distribution. Presently known from several localities in Iran, stretching from the north-eastern Persian Gulf to the northern Gulf of Oman: Khamir, Qeshm Island, Tiab and Guwatr.
Ecology. All specimens were collected close to pneumatophores of mangrove trees. One specimen was found in a small natural puddle, together with the palaemonid shrimp Cuapetes andamanensis (Kemp, 1922) . However, most specimens were found in shovel-dug holes, together with Alpheus aff. euphrosyne De Man, 1897 (Anker, in study) and A. lutosus Anker & De Grave, 2009 , suggesting a possible co-habitation of S. shojaei sp. nov. and one or both of these two much larger snapping shrimps. Remarkably, no specimens of the new species were found in sites that were distant from the mangrove pneumatophores.
Discussion. The new species is a member of the S. gracilipes group according to Anker and Marin’s (2006) tentative subdivision of the genus into seven species groups. The presently known Indo-West Pacific representatives of this group are: S. tafaongae Banner & Banner, 1966 ; S. gracilipes Miya, 1972 ; S. colinorum De Grave, 2004 ; S. alpheophilus Anker & Marin, 2006 ; S. pusillus Anker & Marin, 2006 ; S. falcidactylus Anker & Marin, 2006 ; S. venustus Anker, 2019 ; S. ikaros Anker, Al-Kandari & De Grave, 2020 ; S. rashedi Ashrafi, Ďuriš & Naderloo, 2020 ; S. farasan Anker, 2022 ; and, possibly, S. singularis Komai, Maenosono & Naruse, 2021 . These species are typically encountered on sandy and/or muddy substrates in various habitats ranging from shallow reef flats to subtidal inshore and mangrove flats, sometimes in association with larger burrowing organisms (e.g. De Grave 2004; Anker & Marin 2006; Anker 2019b, 2022; Anker et al. 2020).
Among the Indo-West Pacific members of the S. gracilipes group, S. colinorum appears to be morphologically closest to S. shojaei sp. nov., for instance, in the rostrum being very slender and armed with a subdistal ventral tooth; the unarmed ischia of the major cheliped and fourth pereiopod; the relatively slender dactylus of the third to fifth pereiopods; and the transverse banding of the pleon ( De Grave 2004; Anker et al. 2015; Anker 2019b). However, these two species can be separated by the presence of a distinct rostral carina in S. colinorum (absent in the new species); the unarmed ischia of the minor cheliped and second pereiopod in S. colinorum (both armed with spiniform setae in the new species); and posterior margin of the telson without deep median notch in S. colinorum (with a deep median notch in the new species) (cf. De Grave 2004). The colour pattern of S. colinorum ( Anker 2019b: fig. 6) differs from that of S. shojaei sp. nov. ( Fig. 4 View FIGURE 4 ) in the bands also being present on the carapace, as well as much darker and better defined, and therefore, much more conspicuous in the former species. Noteworthy is that S. colinorum inhabits seagrass flats, often close to mangroves, where it associates with the burrowing snapping shrimps from the Alpheus malabaricus Fabricius, 1775 complex (Anker et al. 2015; Anker 2019b).
The new species can be immediately distinguished from S. alpheophilus , S. pusillus and S. rashedi by the absence of post-rostral tubercle on the carapace, which is present in these three species (albeit small and inconspicuous in some specimens). In addition, S. shojaei sp. nov. may be separated from S. alpheophilus by the unarmed ischium of the major cheliped (vs. armed with a small spinform seta in S. alpheophilus ); the faint banding of the pleon (vs. uniform whitish in S. alpheophilus ); and the green colour of eggs (vs. yellow in S. alpheophilus ) ( Anker & Marin 2006; Anker et al. 2015). However, it must be noted that S. alpheophilus may contain several species (A. Anker, pers. obs.); this taxon is presently being investigated by DNA sequencing. Salmoneus shojaei sp. nov. differs from S. pusillus by the unarmed ischium of the major cheliped (vs. armed with a small spiniform seta in S. pusillus ); the armed ischium of the second pereiopod (vs. unarmed in S. pusillus ); the posterior margin of the telson with a deep median notch (vs. almost straight in S. pusillus ); and the faintly banded colour pattern with green eggs (vs. whitish with yellow eggs in S. pusillus ) ( Anker & Marin 2006). The new species can be easily distinguished from S. rashedi , for instance, by the shorter rostrum; the noticeably more slender telson; the unarmed ischium of the fourth pereiopod (vs. armed with three spiniform setae in S. rashedi ); and in life, also by the very different colour pattern (yelloworange in S. rashedi ) ( Ashrafi et al. 2020).
Salmoneus shojaei sp. nov. differs from S. gracilipes , as described and figured by Miya (1972), by the longer rostrum; the absence of rostral carina (which is well developed in S. gracilipes ); the more posterior position of the dorsal spiniform setae on the telson; and the much weaker low ripples on the ventrolateral surface of the major cheliped palm (which seem to be much more pronounced in S. gracilipes ). As pointed out by Anker et al. (2020), S. gracilipes may represent a species complex. Therefore, all identifications of S. gracilipes after Miya (1972), including the recent records from Kuwait and Indonesia by Anker et al. (2015, 2020), require confirmation. For instance, the Kuwaiti specimen tentatively identified as S. gracilipes presents a small post-rostral tubercle ( Anker et al. 2020: fig. 5A), which was not illustrated for the type specimen (cf. Miya 1972: pl. 3, fig. B).
The most obvious morphological difference between S. shojaei sp. nov. and S. ikaros (presently known only from Kuwait) is the peculiar configuration of the eye cornea in the latter species ( Anker et al. 2020: fig. 1G). Other differences between the two species include the major cheliped ischium unarmed in the new species (vs. armed with a spiniform seta in S. ikaros ); the fourth pereiopod ischium unarmed in the new species (vs. armed with a spiniform seta in S. ikaros ); the noticeably slenderer third pereiopod dactylus; the posterior margin of the telson with a deep median notch in S. shojaei sp. nov. (vs. with a very shallow notch in S. ikaros ); and the presence of transverse banding on the pleon of the new species (vs. pleon without bands in S. ikaros ) ( Anker et al. 2020).
Even though the color pattern of S. venustus ( Anker 2019b: fig. 4) resembles that of S. shojaei sp. nov. ( Fig. 4 View FIGURE 4 ), there are several important morphological differences between these two species. These include, in the new species, the longer and more slender rostrum, reaching to the middle of the second article of the antennular peduncle (vs. reaching only to the distal margin of the first article in S. venustus ); the armed ischia of the minor cheliped and second pereiopods (which are unarmed in S. venustus ); the more slender dactylus of the third pereiopod; and the posterior margin of the telson with a deep U-shaped notch (vs. with a shallow notch in S. venustus ) ( Anker 2019b).
The recently described S. farasan can be separated from S. shojaei sp. nov. using several features of morphology, colour pattern and ecology. Perhaps the most obvious morphological feature to differentiate the new species from S. farasan (cf. Anker 2022: figs. 1, 2) is the noticeably slenderer dactylus of the third to fifth pereiopods; for instance, the ratio dactylus / propodus in the third pereiopod is almost 0.7 in S. shojaei sp. nov. vs. 0.5 in S. farasan . Another difference is the unarmed ischium of the major cheliped in S. shojaei sp. nov. (as opposed to the ischium armed with a spiniform seta in S. farasan ), and the deeper U-shaped notch on the posterior margin of the telson in the new species. In addition, the number of teeth on the fingers of the major chela is around 15 in S. shojaei sp. nov. vs. 20 in the holotype and single known specimen of S. farasan . As mentioned above, S. shojaei sp. nov. has reddish chromatophores on the antennules, anterior part of the carapace, pleon (here forming diffuse transversal bands) and telson, whilst the eggs are greenish. In contrast, S. farasan is an overall whitish shrimp, without discernable red chromatophores, and has orange eggs ( Anker 2022: fig. 3). The two species also differ from the ecological point of view, with the new species being associated with burrows of Alpheus spp. in mangrove forests vs. S. farasan inhabiting coral reefs, where it lives under coral rubble deeply immerged in silty sand ( Anker 2022).
Salmoneus falcidactylus differs from S. shojaei sp. nov. by the extremely elongate, sickle-shaped dactyli of the third to fifth pereiopods, after which the former species was named. Additional distinguishing features of S. falcidactylus are the more acute rostrum; the presence of longitudinal depressions on the dorsal and lateral surfaces of the major cheliped palm; and the unarmed ischia of the minor cheliped and second pereiopods ( Anker & Marin 2006).
The very long and slender rostra of S. tafaongae and S. singularis immediately separate these two species from S. shojaei sp. nov. Salmoneus tafaongae also has much stronger, somewhat up-turned orbital teeth, which is not the case of the new species. In addition, the colouration of S. tafaongae ( De Grave et al. 2020: fig. 5) does not exhibit the faint reddish banding, as seen in S. shojaei sp. nov. Salmoneus singularis can be most easily distinguished from S. shojaei sp. nov. by the position of orbital teeth, which are post-orbital in the former species, representing a unique configuration within the genus Salmoneus ( Komai et al. 2021) .
Three western Atlantic species were originally assigned to the S. gracilipes group: S. armatus Anker, 2010 , S. cavicolus Felder & Manning, 1986 , and S. hispaniolensis Anker, 2010 ( Anker & Marin 2006; Anker 2010a). One of them, S. armatus , was recently transferred, albeit tentatively, to the related genus Triacanthoneus Anker, 2010 , despite the absence of sharp dorsolateral teeth on the carapace, which are characteristic of all other species of the genus ( Anker 2010b, 2020b). The remaining two species remain in the S. gracilipes group for now, although their relationship to the Indo-West Pacific members of the group or other species of Salmoneus remains obscure. Regardless of their phylogenetic affinities, S. shojaei sp. nov. differs from S. cavicolus (holotype only, see Anker 2010a for comments on the type-series) by the rostrum lacking rostral carina (vs. with a short carina in S. cavicolus ), the second article of the antennular peduncle at most 1.2 times as long as wide (vs. 1.8 times as long as wide in S. cavicolus ), and the much longer stylocerite, almost reaching the distal margin of the second article of the antennular peduncle (vs. just overreaching the distal margin of the first article in S. cavicolus ); from S. hispaniolensis by the generally stouter major cheliped, with an unarmed ischium (vs. slenderer and with ischium armed with a spiniform seta in S. hispaniolensis ); and from both of them by the deeply notched posterior margin of the telson (vs. straight in S. cavicolus and S. hispaniolensis ) ( Felder & Manning, 1986; Anker, 2010a).
| R |
Departamento de Geologia, Universidad de Chile |
| FLMNH |
Florida Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |