Macrobrachium tenuipes, Zhu & Chen & Zheng & Chen & Guo, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4759.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:FEDD5D3B-B074-45D1-99C2-757B2938A55E |
|
DOI |
https://doi.org/10.5281/zenodo.3810577 |
|
persistent identifier |
https://treatment.plazi.org/id/8E619D49-C101-44C5-8205-BAA3097E0CB2 |
|
taxon LSID |
lsid:zoobank.org:act:8E619D49-C101-44C5-8205-BAA3097E0CB2 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobrachium tenuipes |
| status |
sp. nov. |
Macrobrachium tenuipes View in CoL sp. nov.
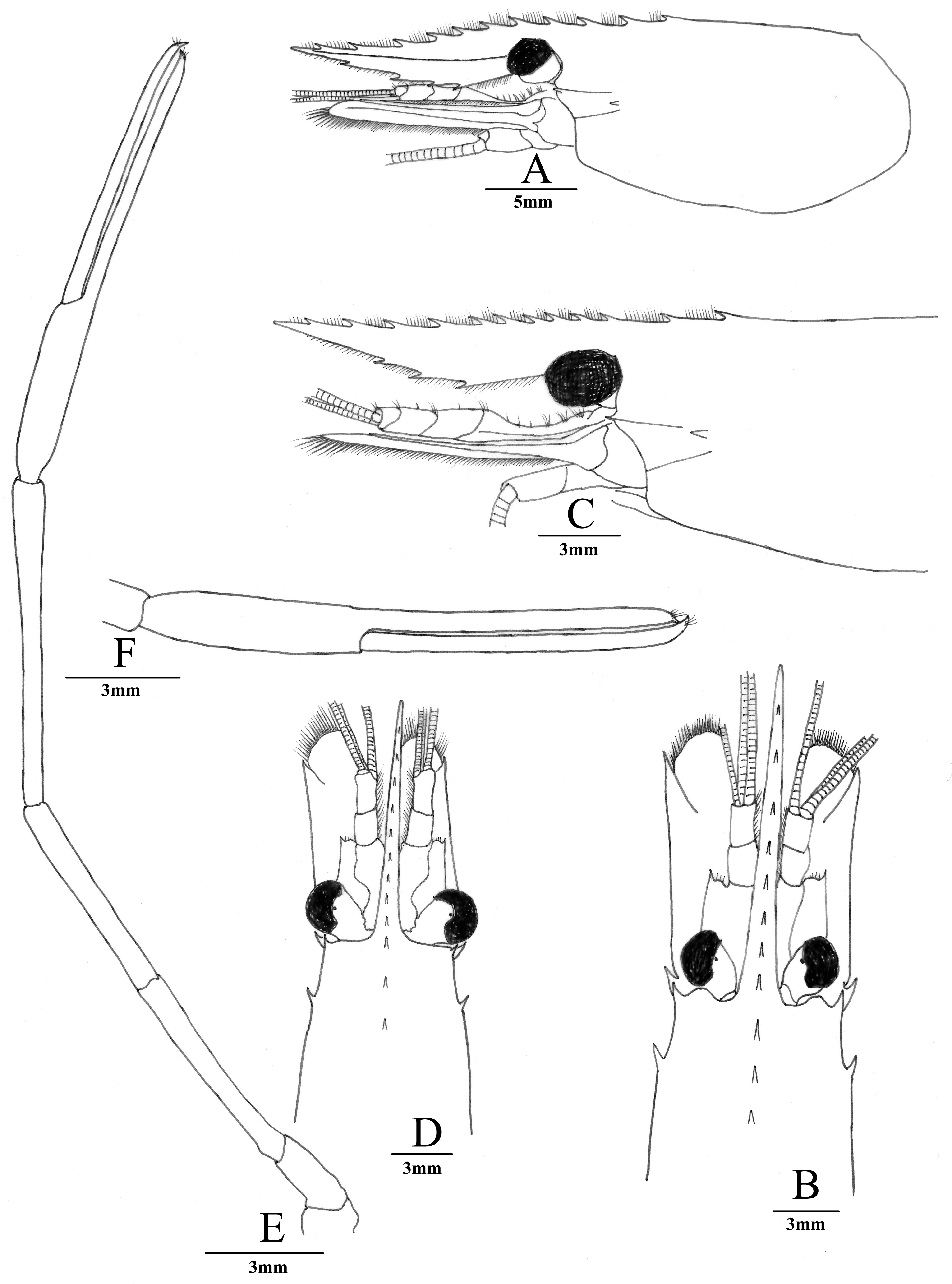
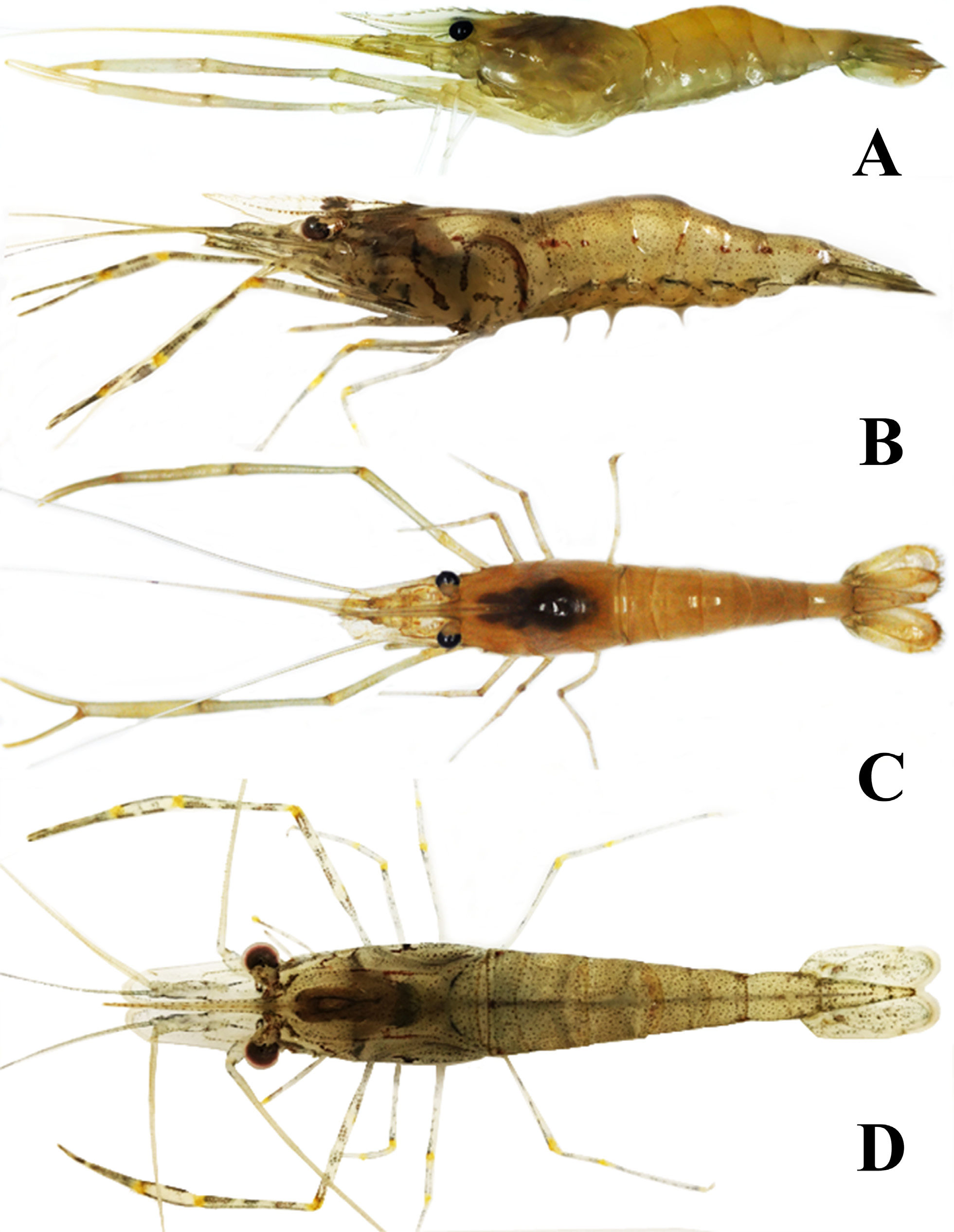
( Figs. 5 View FIGURE 5 , 6 View FIGURE 6 , 7A, B View FIGURE 7 , 8A, C View FIGURE 8 )
Material examined. Holotype: Adult male ( FU, 2018-11-25-01), tl 66.9 mm, cl 17.9 mm, rl 16.5 mm; a cave pool near Lalan , Mashan County, Nanning City , Guangxi Zhuang Autonomous Region, southwestern China ( 23° 41' 42" N, 108° 18' 45" E, alt. 310 m), November 25, 2018 GoogleMaps . Paratypes: 1 male ( FU, 2018-11-25-02) tl 40.8 mm, cl 9.1 mm, rl 8.2 mm . 2 females, ( FU, 2018-11-25-02), tl 60.0– 62.9 mm, cl 15.0– 18.1 mm, rl 14.8–16.0 mm, same data as for holotype GoogleMaps .
Comparative material examined. Macrobrachium superbum (Heller 1862) , 2 males cl 14.5–15.2 mm, 2 females cl 15.8–16.2 mm, Green Cave Pool, near Yanqian Village, Longwen Town, Mei County, Guangdong province, China ( 24° 34' 10" N, 116° 21' 3" E, alt. 105.2m).
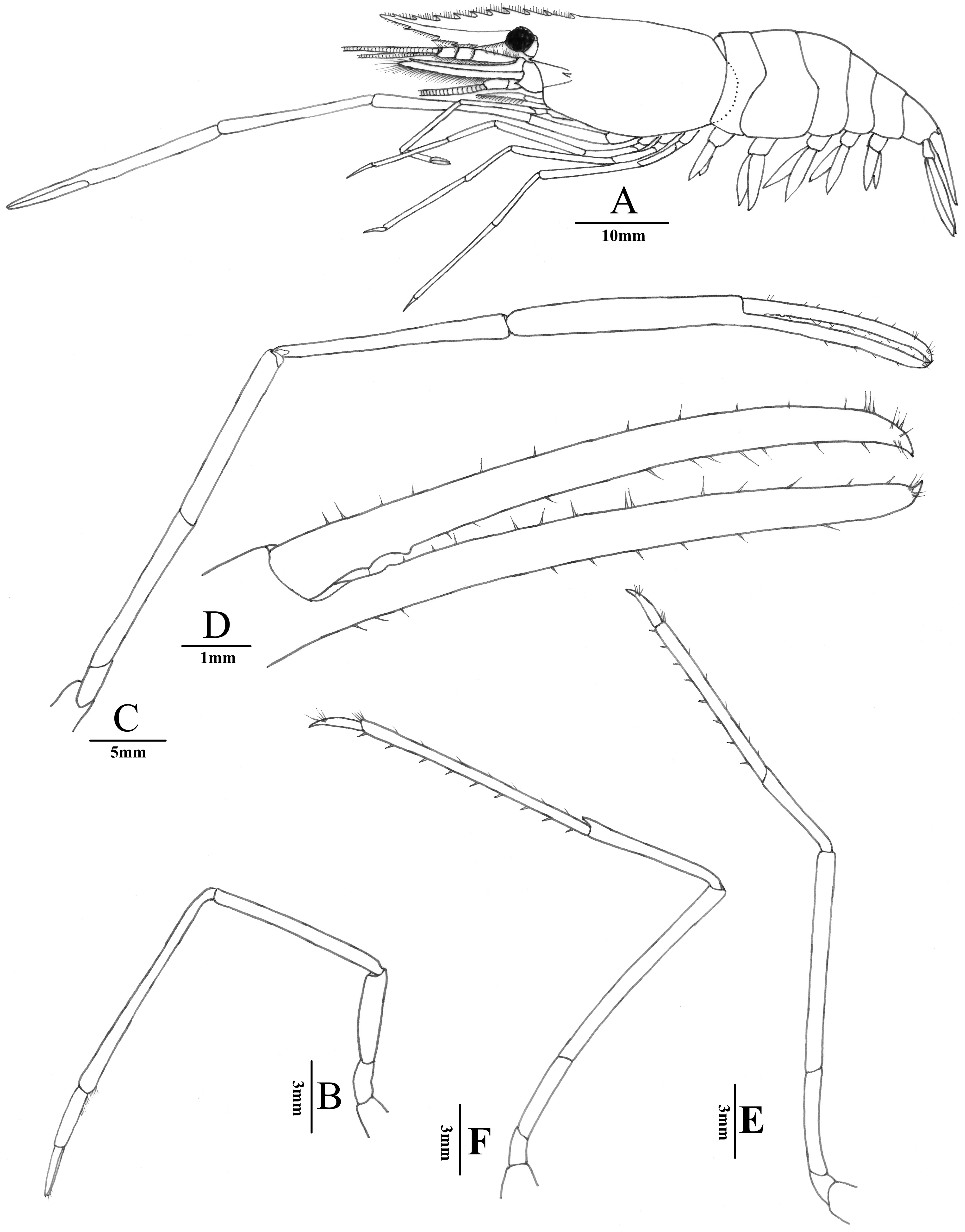
Description. Rostrum ( Figs. 5A View FIGURE 5 , 7A, 7B View FIGURE 7 ) long, slender, directed forward, slightly convex above orbital margin, 0.8–1.1 times of cl, overreaching scaphocerite, directed forward; dorsal margin with 11–12 teeth, of which 3 or 4 teeth behind orbit, these teeth unequally spaced, becoming more widely toward anterior; ventral margin with 3 or 4 teeth.
Carapace ( Figs. 5A View FIGURE 5 , 7A View FIGURE 7 , 8A, 8C View FIGURE 8 ) glabrous,antennal spine well-developed, situated below lower orbital angle, tip slightly overreaching anterolateral margin of carapace; hepatic spine smaller than antennal spine,slightly below level of tantennal spine; branchiostegal groove present.
Eyes ( Figs. 5A View FIGURE 5 , 7A View FIGURE 7 , 8A, 8C View FIGURE 8 ) well-developed;cornea large and pigmented accessory pigment spot evident.
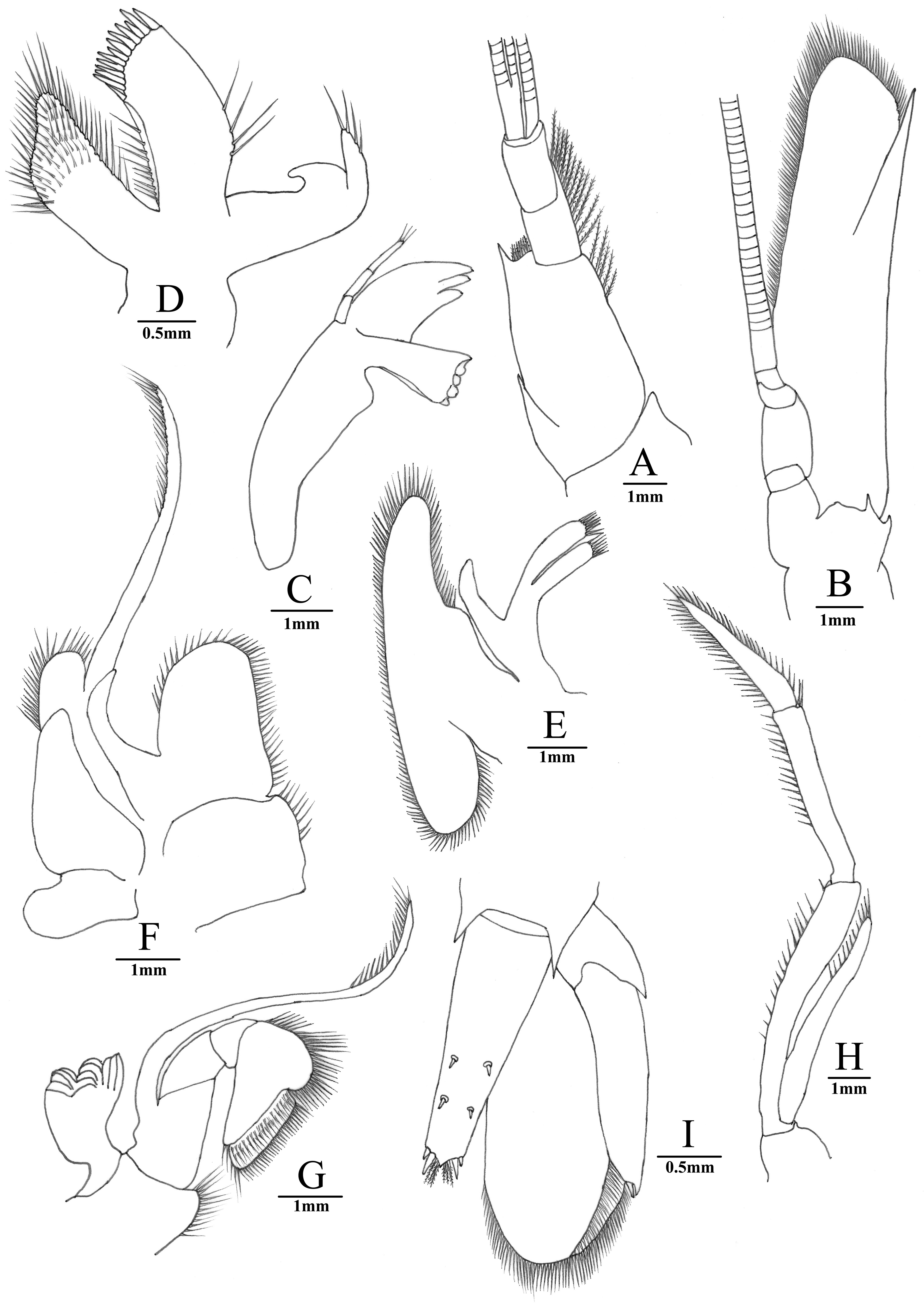
Antennular peduncle basal segment ( Fig. 6A View FIGURE 6 ) with sharp stylocerite reaching midlength of segment; anterior margin of basal segment distinctly convex, distolateral spine slender, reaching beyond half of second peduncular segment; second segment 0.26–0.29 times as long as basal segment, 0.79–0.81 times as long as distal segment. All segments except distal segment with submarginal plumose setae.
Antennal scaphocerite ( Fig. 6B View FIGURE 6 ) relartively slender, subrectangular, 4.1 times as long as wide; outer margin almost straight, terminating in moderately strong spine far exceeded by produced, rounded distal lamella.
Mandible ( Fig. 6C View FIGURE 6 ) with 3-segmented palp; incisor process robust, with 3 sharp teeth; molar process stout, truncate distally, with 3 blunt teeth.
Maxillule ( Fig. 6D View FIGURE 6 ) with bilobed palp, outer lobe stout, slightly longer than lower lobe, setose distally, inner lobe hooked, devoid of setae; basial endite broadly elongated, distal margin with row of strong spines; coxal endite as long as upper lacinia, tapering to truncate tip distally, with dense setae.
Maxilla ( Fig. 6E View FIGURE 6 ) with simple palp; basial endite consisting of two slender lobes, both lobes with numerous simple setae distally; scaphognathite broad, about 4.8 times as long as wide.
First maxilliped ( Fig. 6F View FIGURE 6 ) with distally tapering palp, not reaching anterior margin of caridean lobe, basial and coxal endites distinct, with setae on margin; exopod with well-developed caridean lobe, flagellum with numerous plumose setae distally; epipod divided into two broad lobes, proximal lobe smaller than anterior lobe, oval, distal lobe triangular.
Second maxilliped ( Fig. 6G View FIGURE 6 ) with normal endopod, dactylus fused with propodus for its entire length, with densely setae on margin; exopod well developed, flagellum with plumose setae distally, epipod simple, with welldeveloped podobranch. Most characters described for mouthparts are general for taxa of Palaemoninae .
Third maxilliped ( Fig. 6H View FIGURE 6 ) with moderately slender endopod; antepenultimate with row of simple setae on inner margin; carpus 0.72 times length of antepenultimate, with row of long, simple setae on inner margin and sparse simple setae on outer margin; distal segment about 0.85 times penultimate segment, with rows of long, simple setae on inner and outer margins; exopod not reaching distal end of antepenultimate, with plumose setae distally; two arthrobranchs, one rudimentary and obscured by larger.
First pereiopod ( Fig. 5B View FIGURE 5 ) slender, overreaching scaphocerite by 5/6 length of carpus; ischium 0.52–0.61 times as long as merus; merus 0.76–0.81 times as long as carpus; carpus 1.9–2.1 times as long as the chela; fingers 0.8–0.9 times as long as palm.
Second pereiopods ( Figs. 6C, 6D View FIGURE 6 ) subequal and similar from right to left in both males and females, and extended beyond the scaphocerite by 4/5 the merus.The merus was about 1.1-1.2 times as long as the ischium; carpus 8.9–9.9 times as long as wide, about 1.2–1.3 times as long as merus, 1.1 times as long as palm; palm not particularly inflated, 6.8–7.5 times as long as wide; fingers 0.75–0.86 times as long as palm, not particularly setose, without gape when crossed; fixed finger proximally with 1 tooth on occulsal margin, dactylus proximally with 2 teeth.
Third pereiopod ( Fig. 5E View FIGURE 5 ) relatively slender for genus, overreaching end of third segment of antennular peduncle by 1/2 length of propodus; dactylus 4.6–4.8 times as long as wide, propodus 3.4–3.7 times as long as dactylus, 1.6–1.7 times as long as carpus, with 6–8 spines on flexor margin; merus 1.9–2.1 times as long as carpus. Fourth pereiopod generally similar to third pereiopod, overreaching end of third segment of antennular peduncle by 1/2 propodus.
Fifth pereiopods ( Fig. 5F View FIGURE 5 ) overreaching end of third segment of antennular peduncle by 2/3 length of propodus; propodus 4.5–5.0 times as long as dactylus, 1.5–1.7 times as long as carpus, with 7–9 spines on posterior margin; merus 1.3–1.4 times as long as carpus, dactylus about 5.6 times as long as width, terminating in a small claw.
Male first pleopods with endopod of about half length of exopod; inner margin slightly concave; distal margin rounded, without even trace of appendix interna.
Male second pleopod with well developed appendix masculina on endopod, reaching midlength of endopod, about twice as long as appendix interna, bearing numerous stiff setae.
Abdomen ( Fig. 5A View FIGURE 5 ) glabrous, smooth, pleura of first to fifth somites broadly rounded, sixth somite 1.2–1.3 times as long as fifth somite, about 0.66–0.67 times as long as telson.
Telson ( Fig. 6I View FIGURE 6 ) smooth, 0.42–0.60 times of cl, longer than sixth abdominal segment, dorsal surface with 2 pairs of stout movable spines, posterior margin tapering regularly to a sharp point with 2 pairs of posterior spines, numerous setae present between inner spines.
The uropodal diaeresis ( Fig. 6I View FIGURE 6 ) had a spine, which was slight longer than the outer angle.
Coloration. Body is close to transparency, showing a yellowish color; all appendages generally translucent to faint yellow ( Figs. 8A, 8C View FIGURE 8 ).
Remarks. With regards to the smooth carapace, abdomen, and the relatively slender pereiopods, Phone & Suzuki (2004) referred to Palaemon-like Macrobrachium species group, and referred five species to this group: Arachnochium mirabile (Kemp 1917) (= M. mirabile (Kemp 1917)) , Tenuipedium palaemonoides (Holthuis 1950) (= M. palaemonoides (Holthuis,1950))( Wowor & Ng 2010) , M. superbum (Heller 1862) , M. inflatum Liang & Yan 1985 & M. patheinense Phone & Suzuki 2004 . In addition, the epigean species, M. edentatum Liang & Yan 1986 from Sichuan province and four cave-dwelling speices, include the new species, from Guangxi Zhuang Autonomous Region, i.e., M. lingyunense Li & Luo 2001 , M. elegantum Pan et al. 2010 , M. duanensis Lan et al. 2017 & M. tenuipes sp. nov. also possess slender second pereiopods. All these 10 species can be separated quickly in two subgroups by the ratio of finger and palm of second pereiopod. In the first subgroup ( M. tenuipes sp. nov., M. edentatum , and M. inflatum ), the finger is shorter than the palm. In the second subgroup ( M. superbum , A. mirabile , T. palaemonoides , M. patheinense , M. lingyunense , M. elegantum , and M. duanensis ), the finger is distinctly longer than the palm. The new species, M. tenuipes sp. nov. belongs in the first subgroup. It can be distinguished from M. edentatum by the longer rostrum, beyond end of the scaphocerite (versus reaching the end of third segment of antennular peduncle); presenting proximal teeth on cutting edge of finger of second pereiopod (versus cutting edge entire, lacking tooth), and the merus longer than the ischium (versus the merus shorter than the ischium). M. tenuipes sp. nov. can be distinguished from M. inflatum by with fewer dorsal teeth (8–12 versus 12–17); the palm of male second pereiopod not inflated (versus inflated) and about 6.9–7.4 times as long as wide (versus 3.5–3.6 times); the finger distinctly shorter than merus (versus the finger as along as the merus); and the ischium shorter than the merus (versus the ischium distinctly longer than the merus). Compared to the second subgroup, morphologically, M. tenuipes sp. nov. is more similar to the epigean M. superbum than other 6 species. However, the two species can be easily distinguished because in M. tenuipes sp. nov. the rostrum is slightly convex and with 8–12 dorsal teeth ( Figs. 6A View FIGURE 6 , 7A, 7B View FIGURE 7 ); presenting proximal teeth on the cutting edges of finger of second pereiopod ( Fig. 5D View FIGURE 5 ). Whereas in the M. superbum the rostrum is straight and with 11–15 dorsal teeth ( Figs. 7C, 7D View FIGURE 7 ); lacking tooth on the cutting edges of finger of second pereiopod ( Fig. 7F View FIGURE 7 ). M. tenuipes sp. nov. could be distinguished from M. patheinense by the rostrum is slightly convex, with tip directed forward (versus the rostrum straight, tip slightly upwards), the proportionately shorter carpus of second pereiopod (the carpus is about 1.1 times as long as palm versus about 2.0 timesas long as palm), the ischium shorter than the merus (versus the ischium distinctly longer than the merus), and presenting proximal teeth on the cutting edges of finger (versus lacking tooth on the cutting edges). Regarding to four cave-dwelling speices, M. lingyunense , M. elegantum , M. duanensis , and M. tenuipes sp. nov. from Guangxi, southwestern China. The new species M. tenuipes sp. nov. has well developed eyes, large and pigmented cornea, well accessory pigment spot; shorter finger, slender and not inflated palm of second pereiopod. But other three speices (stygobitic), M. lingyunense , M. elegantum , M. douanensis possess the same characters associated with the subterranean lifestyle, such as, strongly degenerated eyes with unpigmented corneas and the absence of eyestalk; longer finger, and subcylindrical and inflated palm of second pereiopod.
Biotope. Individuals of the present new species were captured at bottom of the darkness cave pool, a atyid shrimp (unidentified), a fish ( Sinocylocheilus macrolepes Wang & Chen 1989), and a crab ( Chinapotamon glabrum Dai et al. 1980 ) were observed and captured simultaneously.
Distribution. Only known from the type locality near Lalan, Mashan County, Nanning City, Guangxi Zhuang Autonomous Region, southwestern China.
Etymology. The species name is derived from the Latin “ tenuis ” meaning slender and “pes” meaning leg in reference to the slender pereiopods.
Sequences alignment and data partition. Both COI and 18S rRNA sequences representing nineteen Macrobrachium species and outgroup were aligned using automatic strategies and default parameters in the MAFFT v7.313 software ( Katoh & Standley 2013). Subsequently, COI and 18S rRNA sequences were concatenated. Final alignment length reached up to 1216bp within which COI sequences were accounted within the first 543 basepairs and 18S rRNA sequences constituted basepairs from 544 to 1216. The best model of evoluton was GTR+G model for each data block.
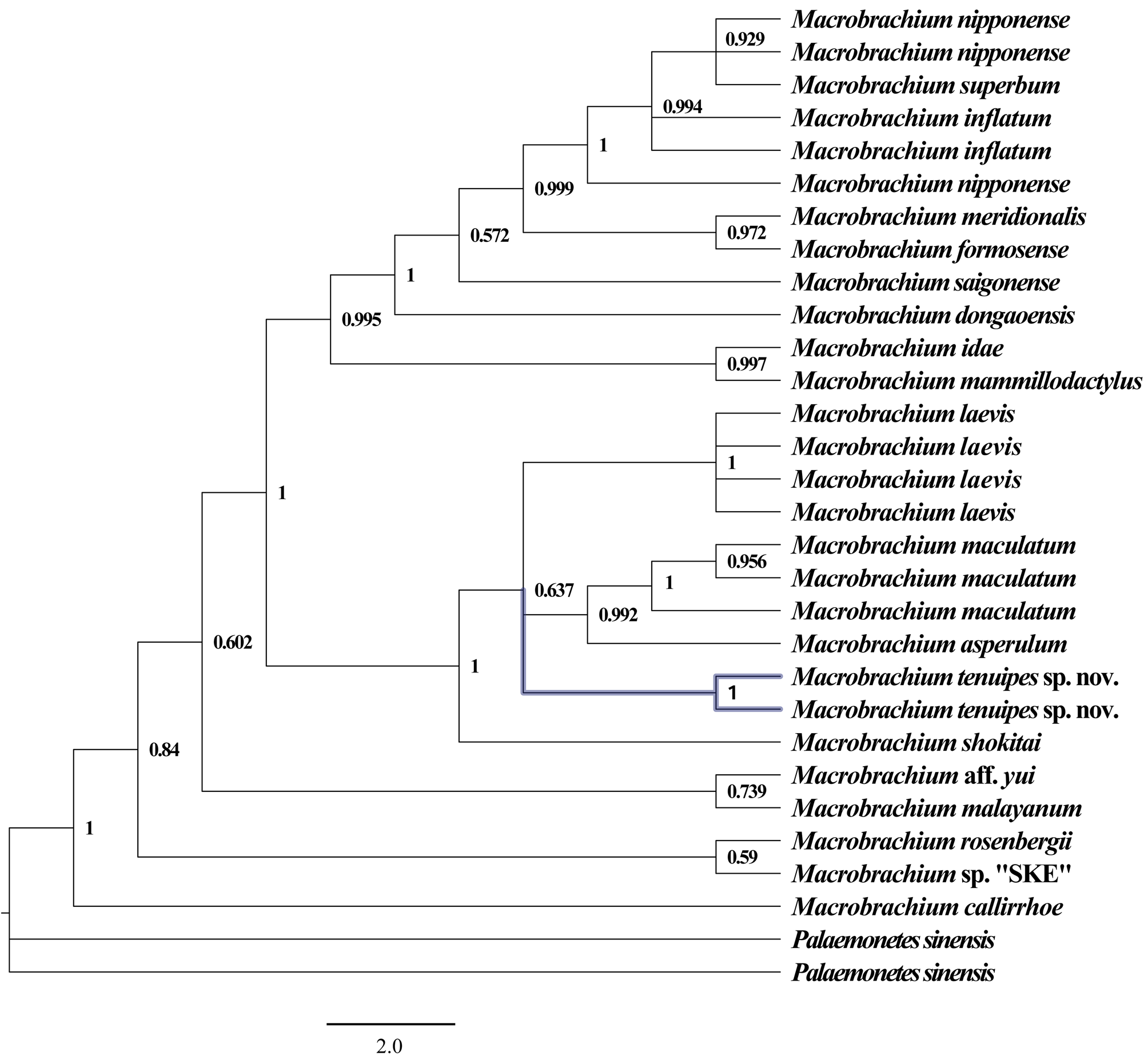
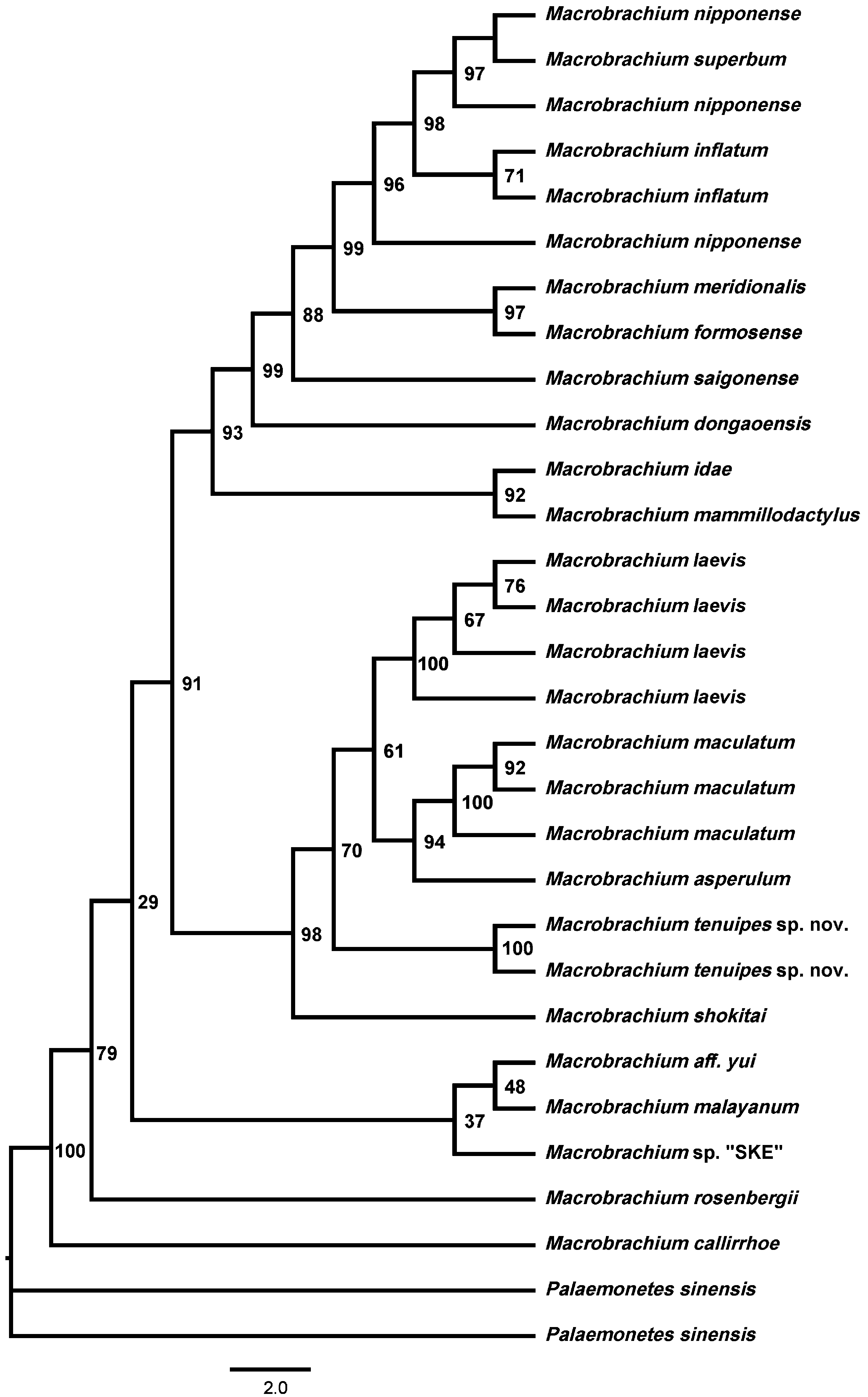
Molecular phylogenetic results. The topology of the Bayesian method (BI) tree and the maximum likelihood method (ML) tree are basically the same. Phylogenetic tree revealed the relationship between Macrobrachium tenuipes sp. nov. and other nineteen species of palaemonids. M. tenuipes sp. nov. is clustering with M. laevis , M. maculatum and M. asperulum ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ). The genetic distance between M. tenuipes sp. nov. and the remaining eighteen species of Macrobrachium based on COI and 18S rRNA are indicated in Table 2. The new species is well isolated from M. superbum with a sequence divergence of 15.5% (COI) and 2.9% (18S rRNA), respectively, supporting the morphology-based description of M. tenuipes as a new species.
Interspecific genetic divergence (K2P) among these nineteen species were 9.4%–23.7% (COI) and 0.9%–4.9% (18S rRNA), respectively. Based on COI sequence, the genetic distance of Macrobrachium tenuipes sp. nov and the remaining eighteen of the Macrobrachium in this study was greater than 2%. This result is in accordance with the minimum interspecific genetic distance of 2% recommended by Hebert, Ratnasingham & de Waard (2003). In the 18S rRNA pairwise genetic distance, in addition to the genetic distance between M. tenuipes sp. nov. and M. asperulu m and M. maculatum is slightly less than 2%, the genetic distance from the other Macrobrachium is greater than 2%. It can be seen from the morphological description that the new species is the most similar in morphology to the M. superbum . In the DNA barcode technology framework, there are two criteria for species definition ( Wiens & Penkrot 2002; Hebert et al. 2004): (1) a standard genetic distance threshold for distinguishing between species and interspecies variability is called “barcode gap”; (2) Species form separate branches in the phylogenetic tree. The "barcode gap" was successfully detected in the DNA barcode fragment used in this study. Based on genetic data, Macrobrachium tenuipes sp. nov. and M. superbum have a large interspecific genetic distance (15.5% in COI and 2.9% in 18S rRNA). It is very useful to study the relationship between intraspecific population and related species by studying mitochondrial DNA. The rate of evolution of the rRNA gene is much slower. In addition, the new species also formed an independent branch. In the phylogenetic tree ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ), Macrobrachium tenuipes sp. nov. (two specimens) form a clade with M. laevis , M. maculatum and M. asperulum , with this clade being a sister to M. shokitai . The posterior probabilities and bootstrap are 63.7% and 70%, Moreover, the topological structure of the NJ tree and the ML tree are basically the same, and in both phylogenetic trees, Macrobrachum tenuipes sp. nov. can form an independent branch. Therefore, here we have reason to believe phylogenetic analyses of the mtDNA COI sequence and nuclear genome 18S rRNA confirmed that M. tenuipes could be identified as a new species.
| FU |
Fudan University, Department of Biology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Caridea |
|
Family |
|
|
Genus |