Hipposideros kingstonae, Wongwaiyut & Karapan & Saekong & Francis & Guillén-Servent & Senawi & Khan & Bates & Jantarit & Soisook, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5277.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:83191D39-BF28-42ED-A31E-112FC3BB524C |
|
DOI |
https://doi.org/10.5281/zenodo.7893587 |
|
persistent identifier |
https://treatment.plazi.org/id/E602879B-FFD1-FFD4-FF7A-FF4BFBC2FB87 |
|
treatment provided by |
Plazi |
|
scientific name |
Hipposideros kingstonae |
| status |
sp. nov. |
Hipposideros kingstonae sp. nov.
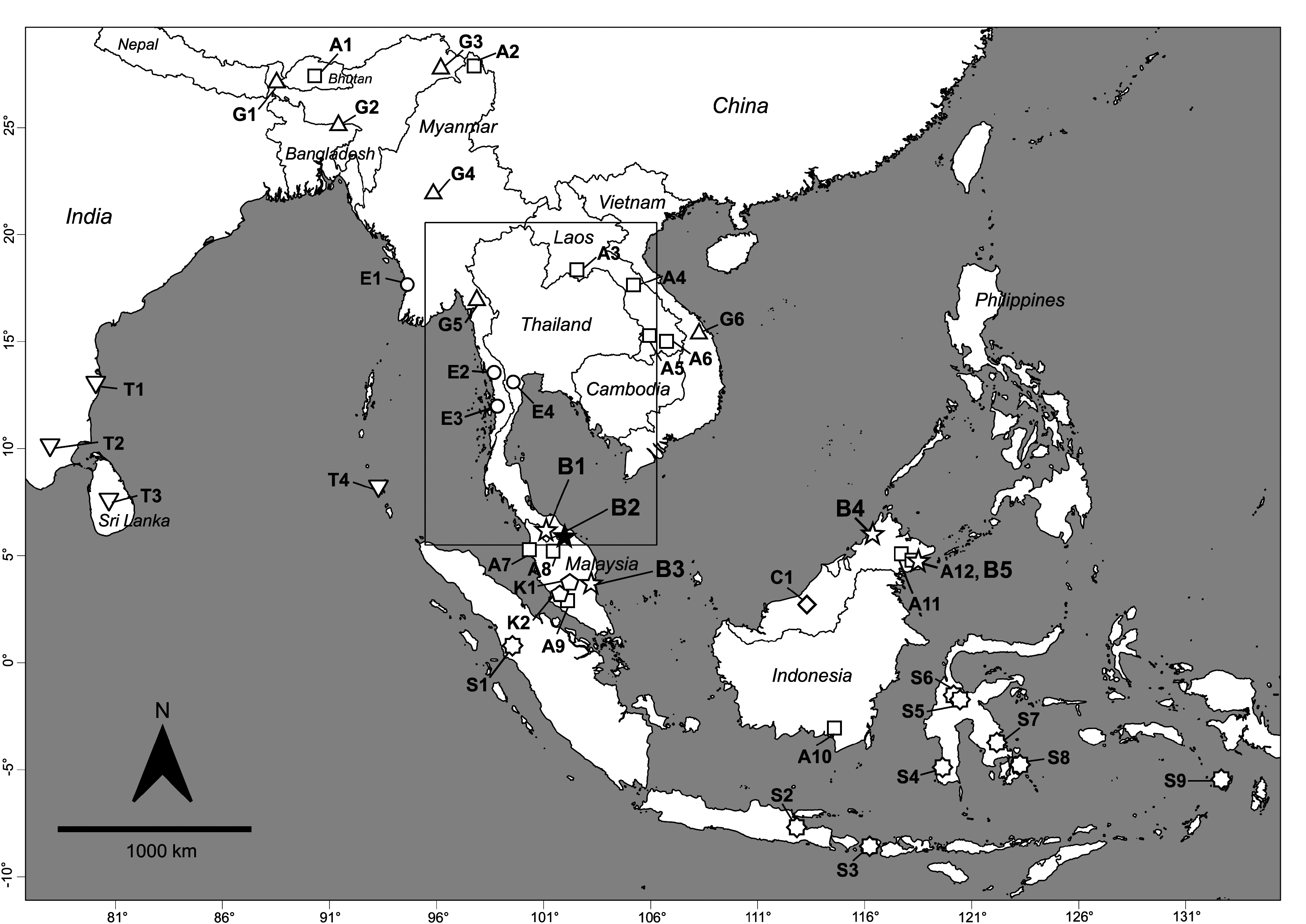
Figs. 3–6 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 9 View FIGURE 9 ; Tables 1–2 View TABLE 1 View TABLE 2
[= H. cineraceus- B ( Kingston et al., 2006; Murray et al., 2012; 2018)]
Holotype. PSUZC-MM2014.164 (field number PS140903.2), adult male, body in alcohol, skull and baculum extracted, collected on 3 September 2014, by Sunate Karapan, Puchit Saekong and Pipat Soisook.
Full measurements (in mm) of the holotype are as follows; FA: 38.4, HB: 40.2, E: 14.2, Tail: 25.9, TIBIA: 16.7, HF: 6.81, GTL: 16.35, SL: 16.13, CBL: 14.54, CCL:13.94, ZB: 8.20, BB: 7.66, MW: 8.50, PC: 2.75, C–M3: 5.54, C1–C1: 3.25, M3–M3: 5.83, c–m3: 5.88, ML: 9.62, ALSW: 4.18, AMSW: 2.19.
Type locality. Phru To Daeng Peat Swamp Forest (= Sirindhorn Peat Swamp Forest), Princess Sirindhorn Wildlife Sanctuary, Narathiwat Province, Thailand, 6°4’ N, 101°58’ E, 170 m a.sl. The specimen was collected in a harp trap in combination with a mist net set along a boardwalk in the swamp forest GoogleMaps .
Paratypes. Thailand — PSUZC-MM2022.2 (field number PS211113.5), adult male ♁, body in alcohol, skull and baculum extracted, collected on 13 November 2021 by Sunate Karapan, Phutita Wongwaiyut and Pipat Soisook from the same area as the holotype but in a different spot, near the edge of the forest ( 6°4.3’ N, 101°57.8’ E). It was caught in an 18 m-long mist net along a boardwalk by the edge of the swamp GoogleMaps . PSUZC-MM2022.1 (field number BL160219.1), adult female, body in alcohol, skull extracted, collected by Puchit Saekong and Sunate Karapan from the same locality as the holotype on 19 February 2016 GoogleMaps .
Referred specimens. Thailand — PSUZC-MM2014.165 (field number PS140830.2), adult female, body in alcohol, skull extracted, collected from Border Police Base, Bang Lang Dam, Hala-Bala Wildlife Sanctuary, Yala Province, 6°4’ N, 101°17’ E, 22 m a.s.l., on 30 August 2014, by Sunate Karapan, Puchit Saekong and Pipat Soisook GoogleMaps . Malaysia — Uncatalogued specimen field number TK020622.10, adult female, body in alcohol, skull extracted, collected from Lubuk Baung, Krau Wildlife Reserve , Pahang, peninsular Malaysia, on 22 June 2002, by Juliana Senawi. This specimen was caught in a four-bank harp trap set across a forest trail in lowland dipterocarp forest. DWNP-M- 1996-07-29 -05653 (field number TK960729.1), adult male, body in alcohol, skull extracted, collected from Kuala Lompat, Krau Wildlife Reserve , Pahang, peninsular Malaysia, on 29 July 1996 by Tigga Kingston. This specimen was caught in a four-bank harp trap in a lowland dipterocarp forest trail. DWNP-M- 1996-05-12 - 05654 (field number TK960519.1), adult male, body in alcohol, skull and baculum extracted, collected from Kuala Lompat, Krau Wildlife Reserve , Pahang, peninsular Malaysia on 19 May 1996 by Tigga Kingston. This specimen was caught in a four-bank harp trap in a lowland dipterocarp forest trail. SMF83823 About SMF (field number CMF920706- 01 ), immature male, collected from Krau Wildlife Reserve , Pahang, peninsular Malaysia on 5 July 1992 by Charles M. Francis. EBD 23565 View Materials (field number 960523n05), adult female, EBD 23561 View Materials (field number 960523n06) and EBD 23560 View Materials (field number 969523n33), adult males, previously identified as H. cf. cineraceus , caught together with 6 other individuals ( 3 female, 3 male) in a four-bank harp trap set in a lowland forest trail about ~150 m from the entrance of Madai Caves, Sabah by Antonio Guillén-Servent and Charles M. Francis. EBD 23821 View Materials (field number 960604n01), adult female, previously identified as H. cf. cineraceus , caught in a mist-net set in the understory of the lower montane forest near the park headquarters in Gunung Kinabalu National Park, Sabah by Antonio Guillén-Servent .
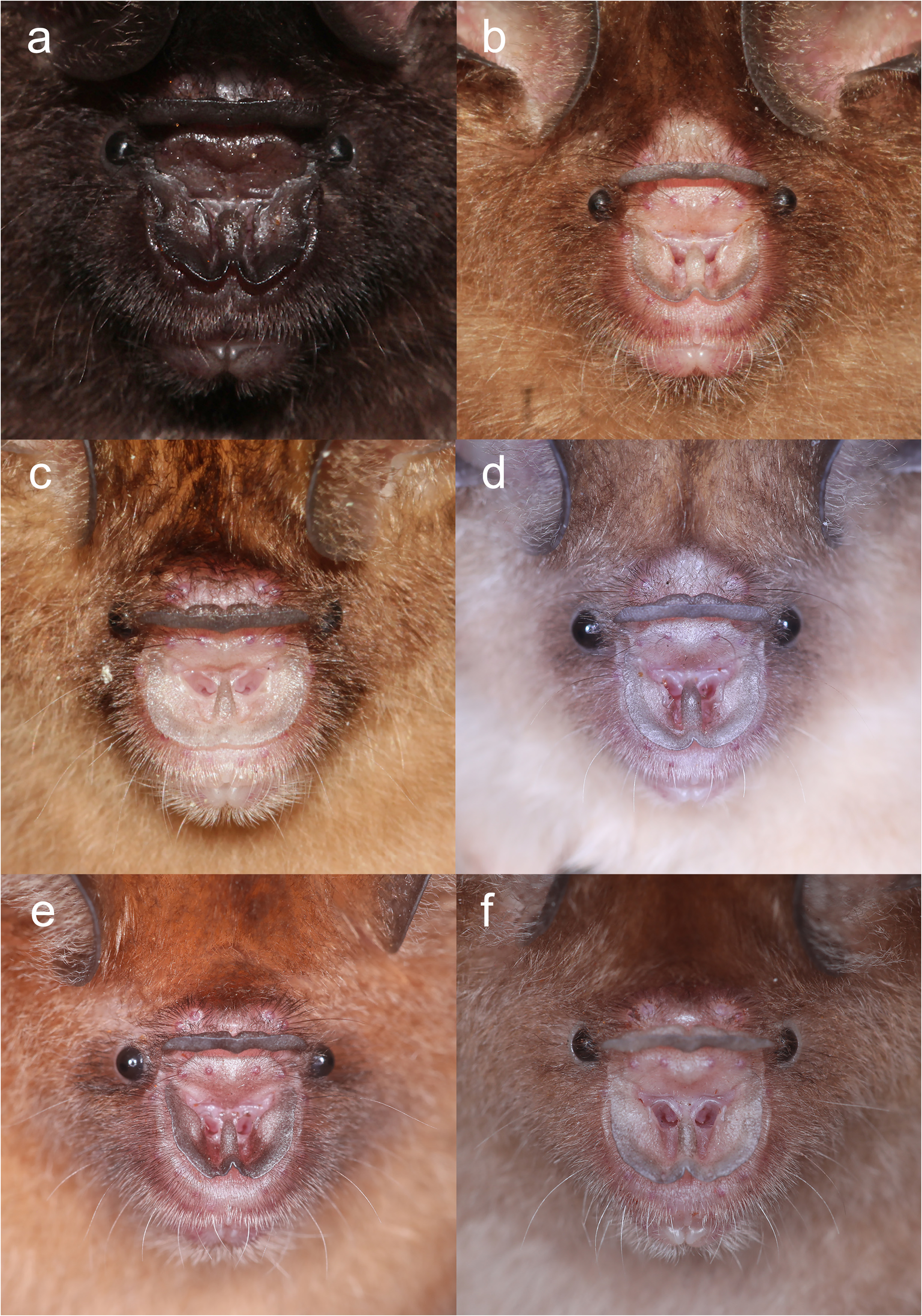
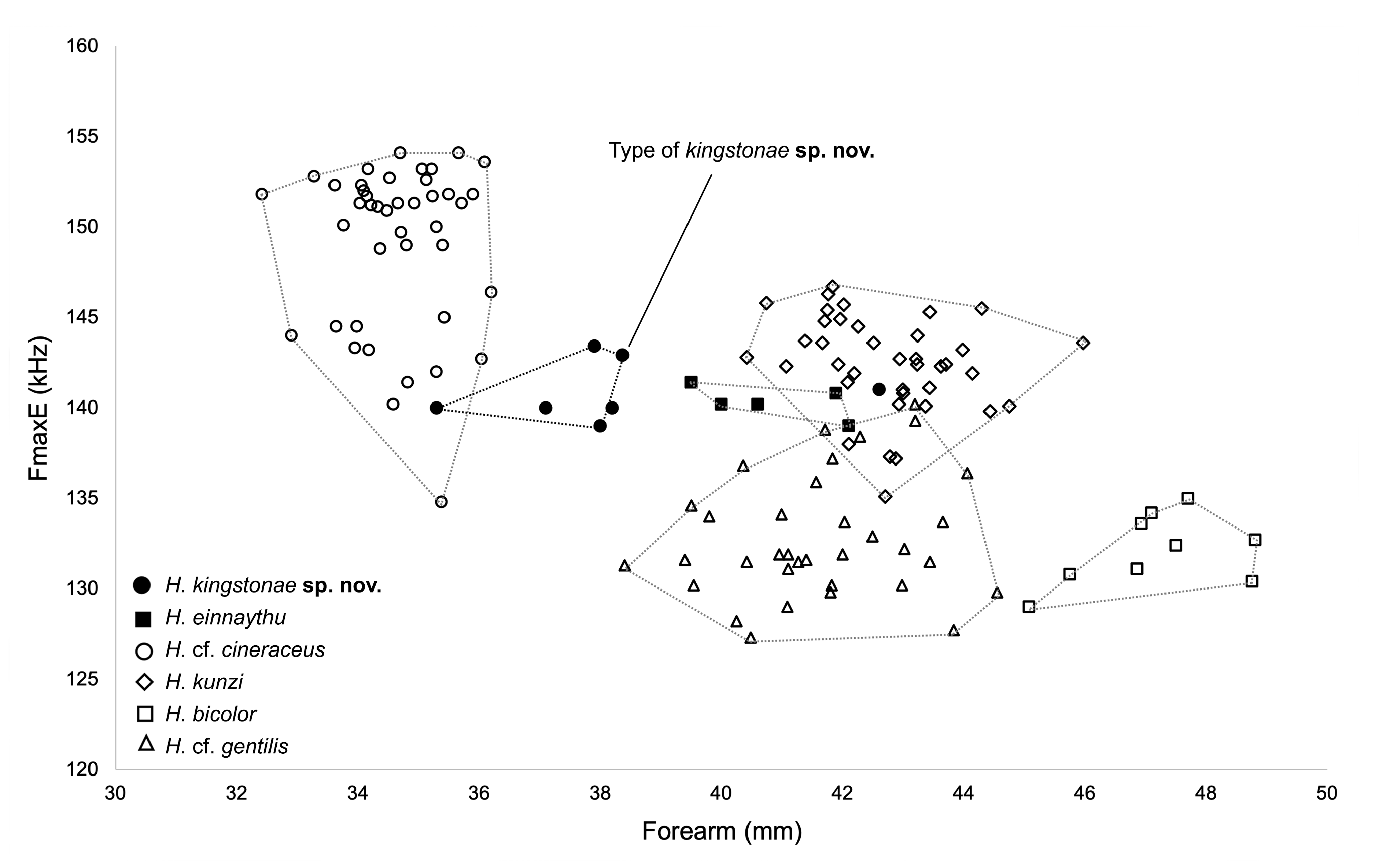
Diagnosis. This is a small Hipposideros with a FA of 35.3–42.6 mm and GTL of 15.94–17.90 mm. The sides of the anterior part of the noseleaf are slightly concave; the anterior border has a deep V-shaped median notch and is somewhat angular in appearance. The internarial septum is large, rounded, and distinctly swollen from the middle to the top. The lateral leaflet is absent. The dorsal pelage is dark brown, with the individual hairs creamy-white from the base to the mid-part. The ventral pelage is orange-brown; the hairs are dark brown at the tip, and paler at base. The baculum is very small, 0.5 mm in length, with short but distinct distal prongs. The constant frequency (CF) element of the echolocation call is 141.0–144.0 kHz in the Thai-Malay Peninsula individuals and 132.3-141.4 kHz in the Bornean individuals.
Etymology. The species is named in honour of Tigga Kingston, who as the chair and founder of the Southeast Asian Bat Conservation Research Unit (SEABCRU), spearheads the global bat research community in understanding diversity and promoting bat conservation.
Description. Hipposideros kingstonae sp. nov. is a small hipposiderid with a forearm length of 35.3–42.6 mm ( Table 1 View TABLE 1 ). The body mass is 4.9–7.0 g (n=4), with three male specimens at 4.9–5.6 g, and one female at 7.0 g. The ear is rounded with a pointed tip, and a height of 14.0– 18.7 mm; it has short brown hairs along the inner sides. The tail is relatively short (21.5–30.0 mm) in comparison to the head and body length (40.2–49.0 mm). The hindfoot is shorter than half of the tibia in length, 5.0– 7.1 mm versus 14.7–17.9 mm, respectively.
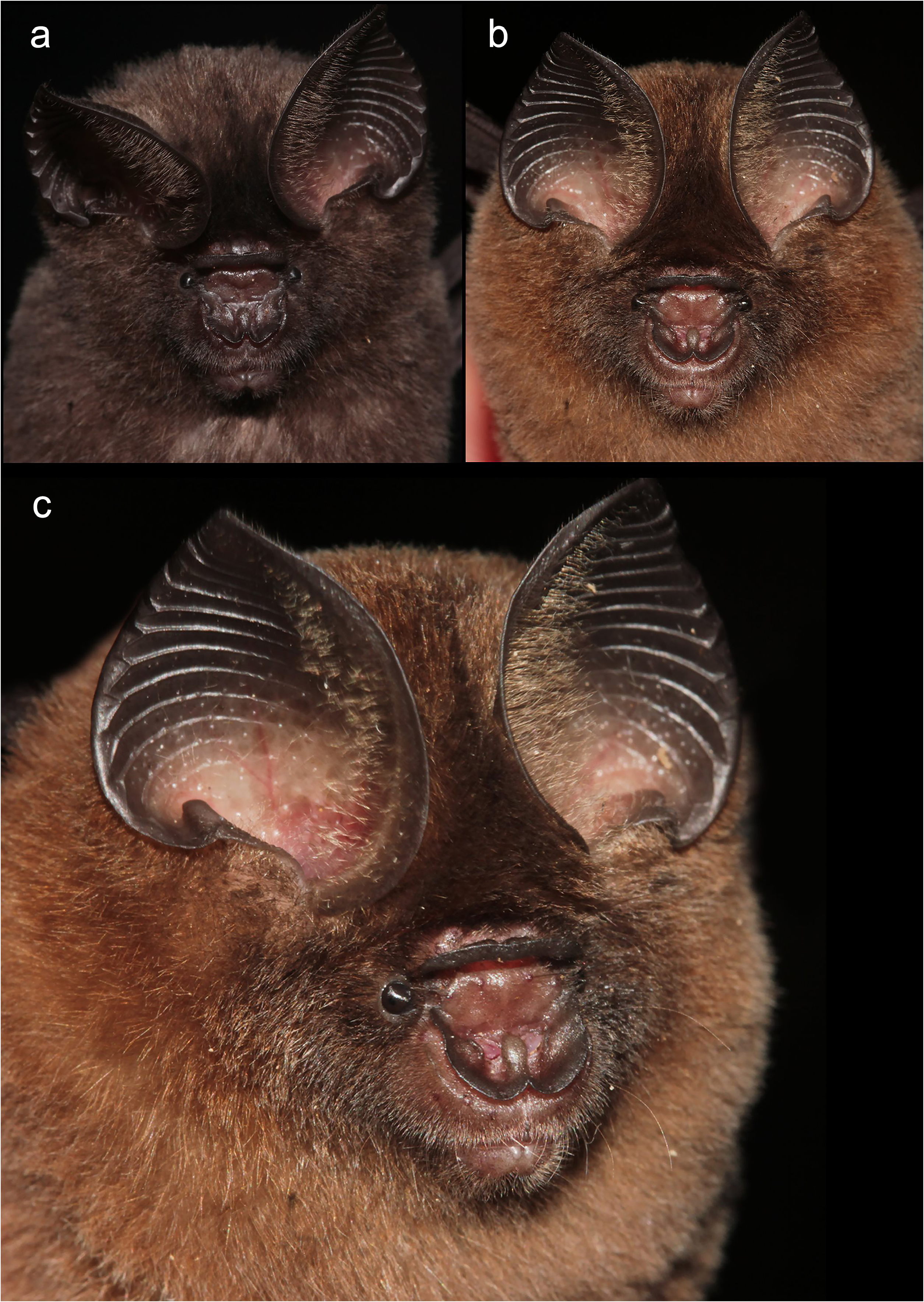
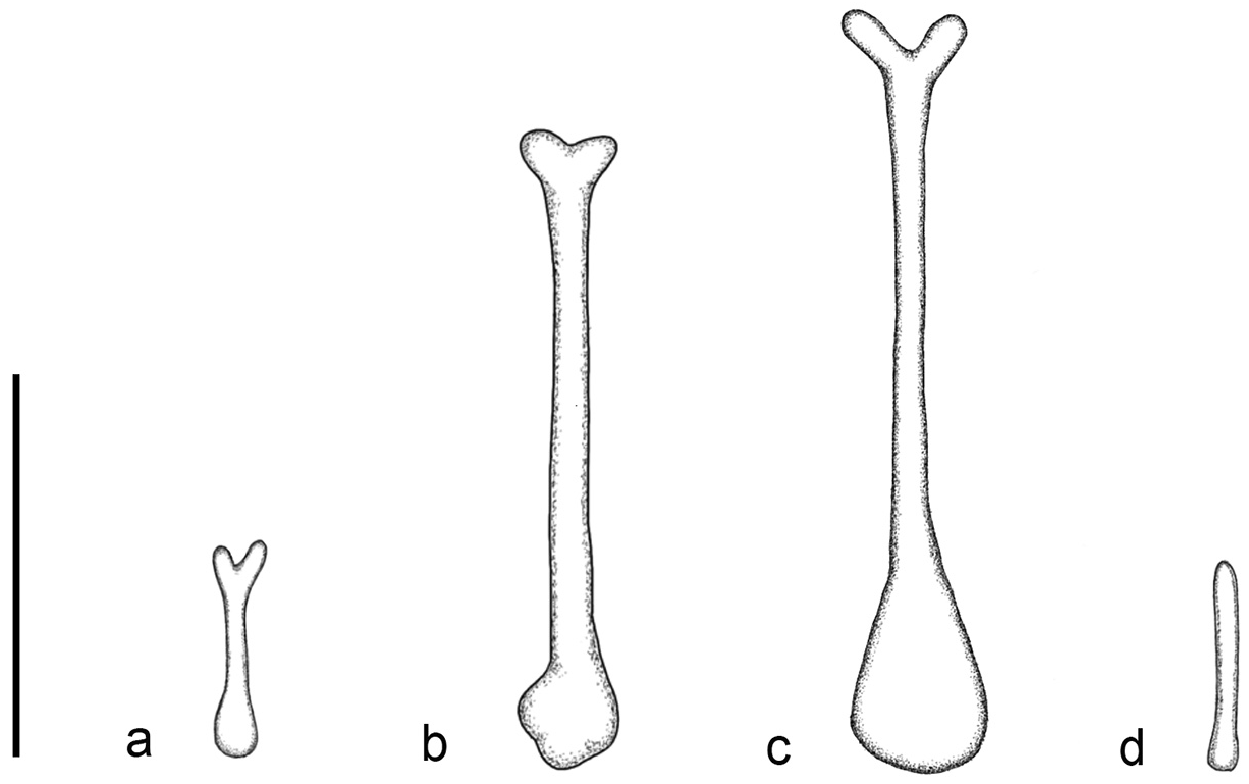
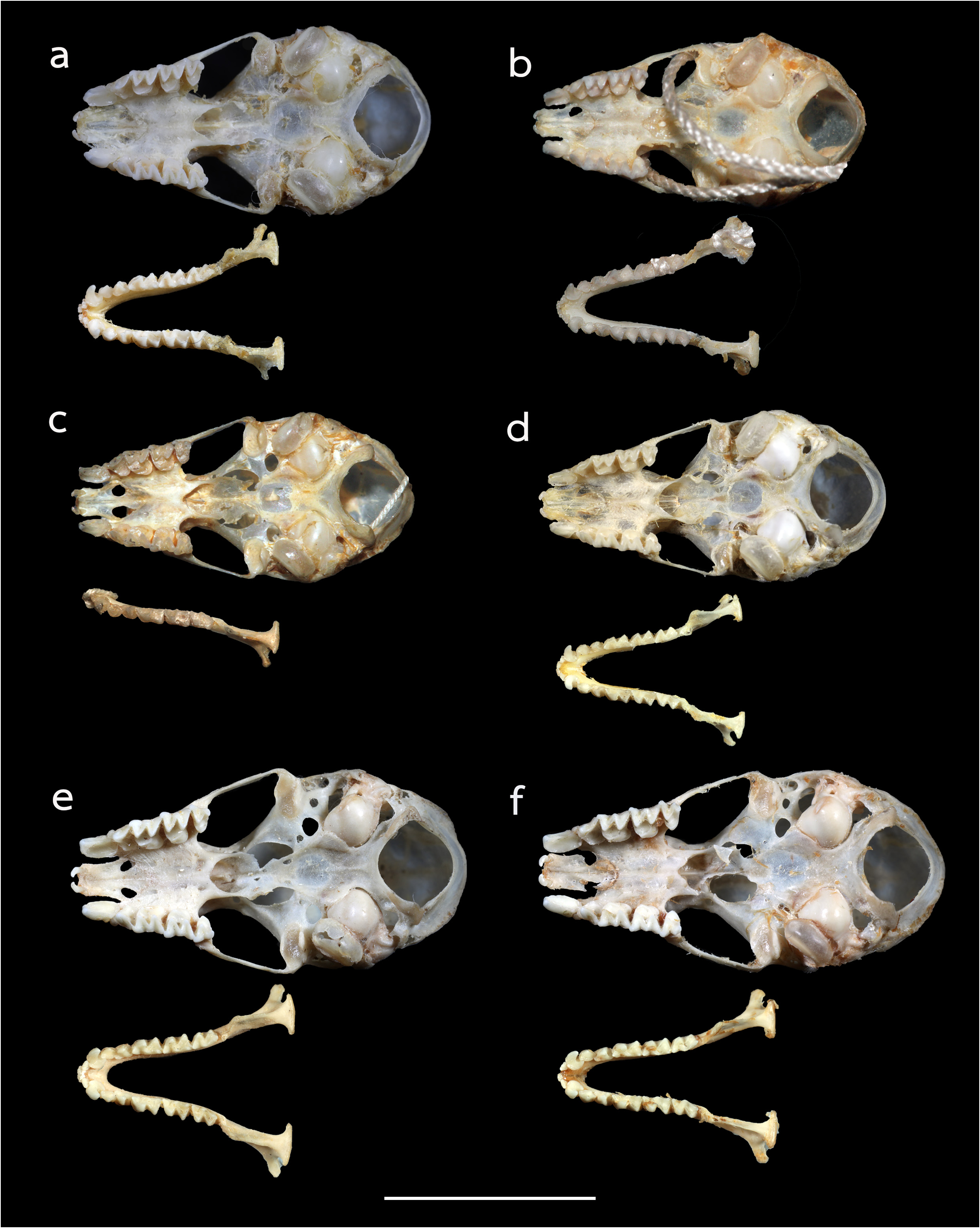
The noseleaf is without a lateral supplementary leaflet ( Fig. 3 View FIGURE 3 ). The anterior leaf is dark brown, slightly concave on both lateral borders. The anterior border (bottom edges) has an angular appearance and is separated by a deep groove in the middle. The internarial septum is large, rounded, and distinctly swollen from the middle to the top. The intermediate leaf is relatively broad and sparsely haired. The male specimens have a well-defined frontal sac behind the posterior leaf. The dorsal pelage is dark brown ( Fig. 3 View FIGURE 3 ), with the base to the middle of the hairs creamy white. The ventral pelage is orange brown to dark brown at the hair tips, and paler at the bases. The baculum is very short, 0.5 mm in length (n=3) ( Fig. 4a View FIGURE 4 ). The shaft is narrow and straight in dorsal and ventral view, with a rounded base and bifid tip. In lateral view, it is slightly curved from the middle towards the base.
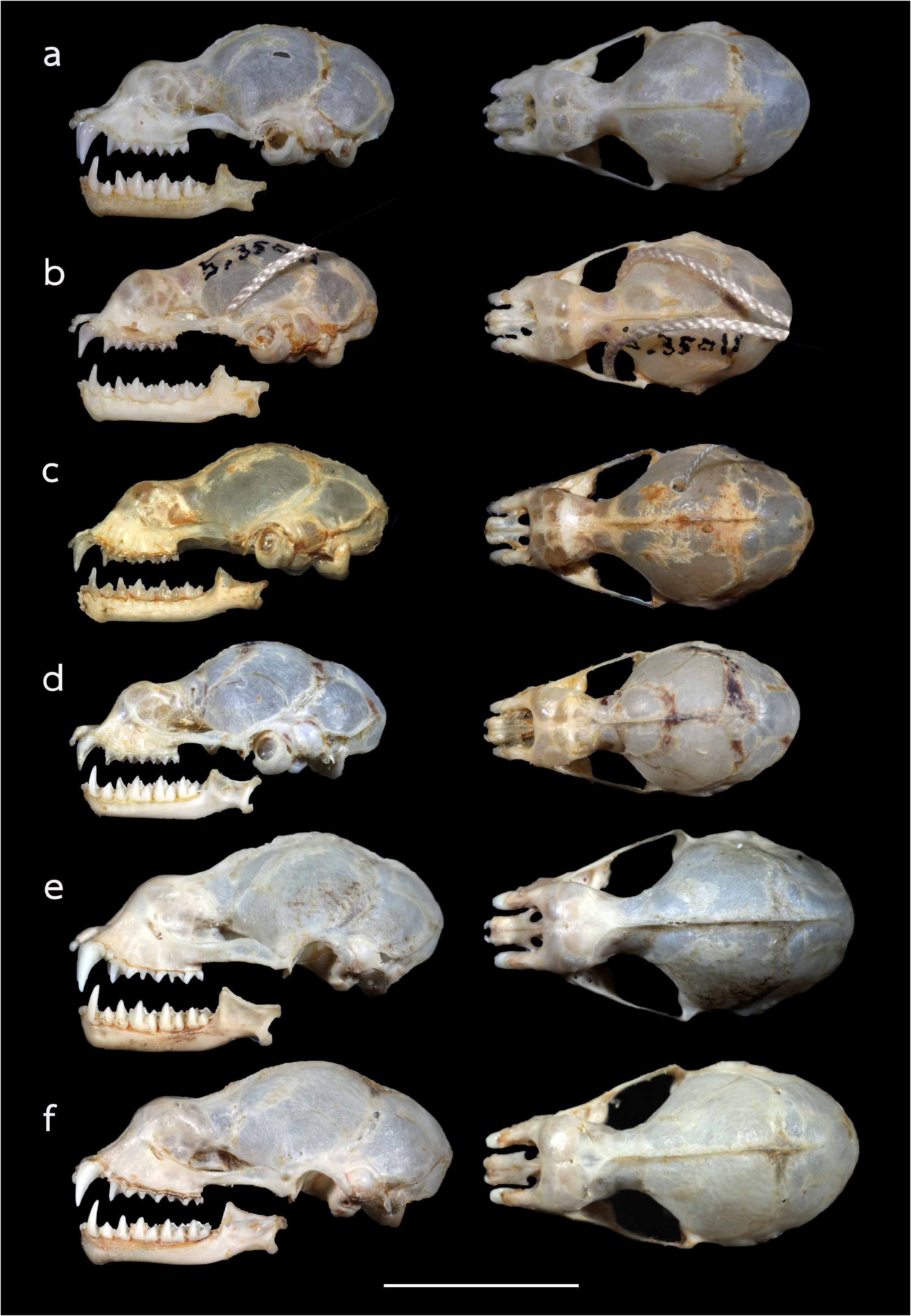
The skull is elongate, with a mean greatest length of the skull (GTL) of 16.29 mm ( 15.94–17.90 mm), a skull length (SL) of 16.13 mm ( 15.73–17.79 mm) and a condylocanine length (CCL) of 14.00 mm ( 13.56–15.94 mm) ( Table 2 View TABLE 2 ). The mastoid width (MW) is 8.32 mm ( 8.07–8.50 mm). This slightly exceeds the zygomatic breadth (ZB), which is 8.01 mm ( 7.79–8.26 mm). In lateral view, the nasal swelling is well-developed, and the sagittal crest is clearly defined particularly on the anterior part of the braincase ( Fig. 5a View FIGURE 5 ). The anterior median swellings are rounded, with the AMSW 3.07 mm ( 1.99–4.28 mm) in width. The frontal depression is shallow when viewed from either the side or the top ( Fig. 5a View FIGURE 5 ). The postorbital constriction (PC) is 2.62 mm ( 2.40–2.91 mm). The zygomata are narrow with an angular process projecting upwards in the mid-part of the jugal bone ( Fig. 5a View FIGURE 5 ). The upper canine (C1) is large, about twice the height of the second upper premolar (P4). The crown area of the P4 is about twothirds that of the C1 ( Fig. 6a View FIGURE 6 ). The first upper premolar is very small and fully extruded, so that the C1 and P4 are in contact. The upper toothrow length (C–M3) is 5.41 mm ( 5.12–5.81 mm). The lower toothrow length (c–m3) is 5.67 mm ( 5.05–6.28 mm) and the mandible length (ML) is 9.64 mm ( 9.22–10.92 mm) ( Table 2 View TABLE 2 ). The lower canine (c1) has an elongated postero-basal heel ( Fig. 6a View FIGURE 6 ). The c1 is twice the height of the second lower premolar (p4). The p4 is rounded and subequal to that of the c 1 in crown area. The first lower premolar (p2) is short and only about half the height of (p4) ( Fig. 5a View FIGURE 5 ).
Echolocation. The echolocation call of H. kingstonae sp. nov. is a typical CF-FM signal. In three individuals recorded from Thailand, the frequency of maximum energy (FmaxE), corresponding to the frequency of the constant element (CF), is 142.4 kHz (141.0–143.4 kHz; n=3). In peninsular Malaysia, the FmaxE from Krau Wildlife Reserve is 144.0 kHz (n=1), whereas in Malaysian Borneo, the FmaxE of the bats from the Madai caves was 138.4 kHz (136.6-141.4 kHz; n=5 individuals) for males and 137.7 kHz (137.4–138.2 kHz; n=4 individuals) for females, while the female from Gunung Kinabalu had a FmaxE of 132.3 kHz.
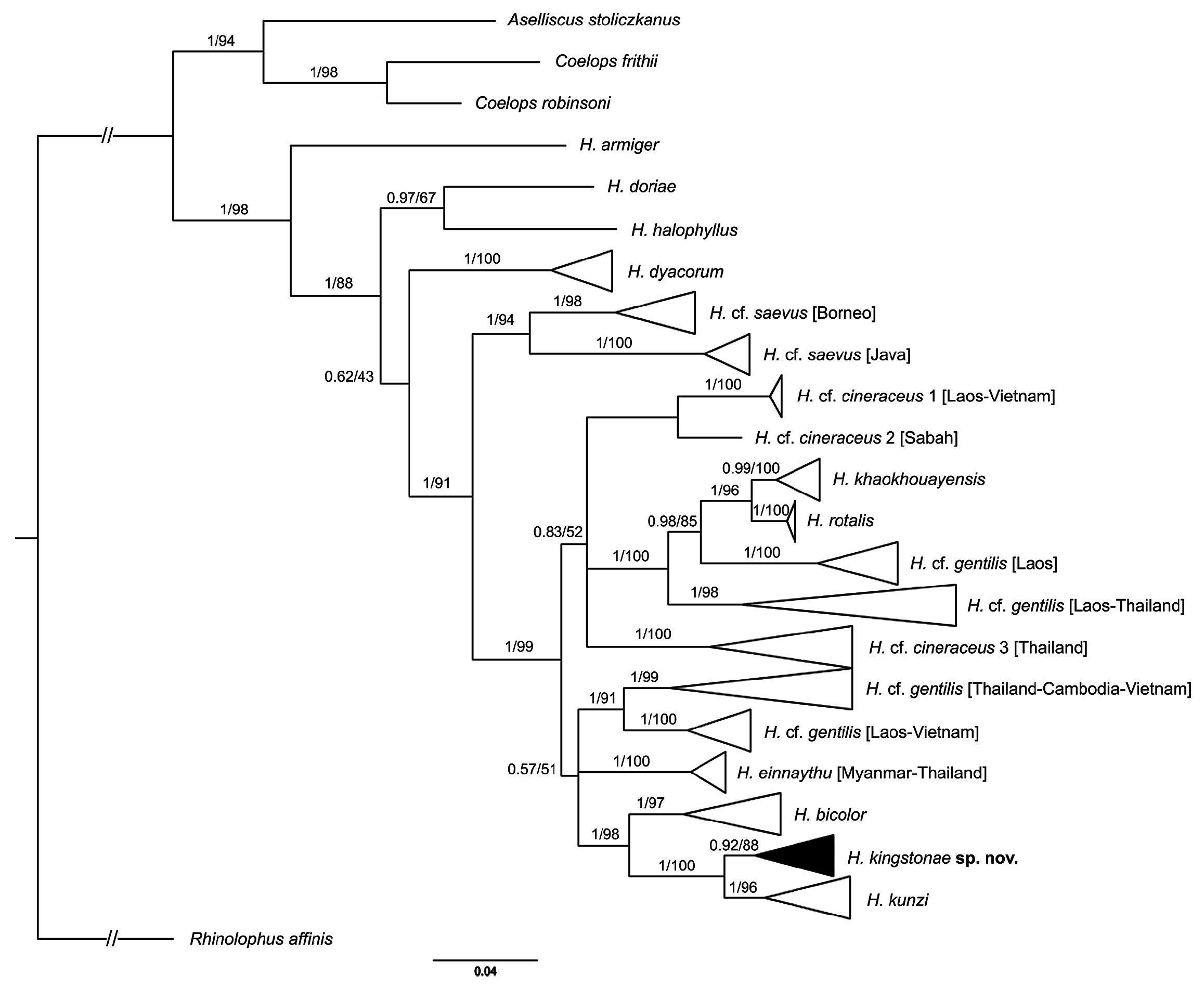
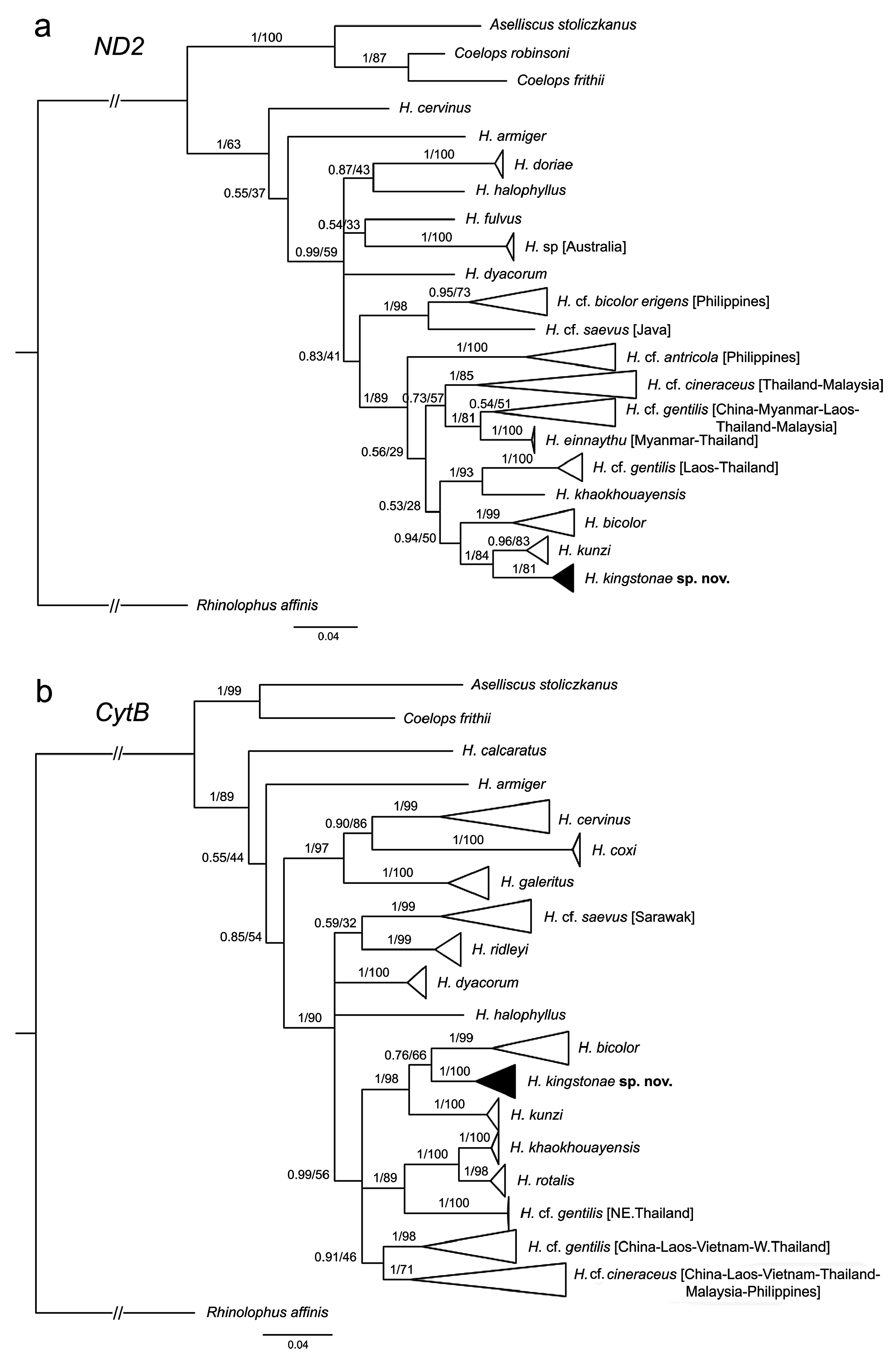
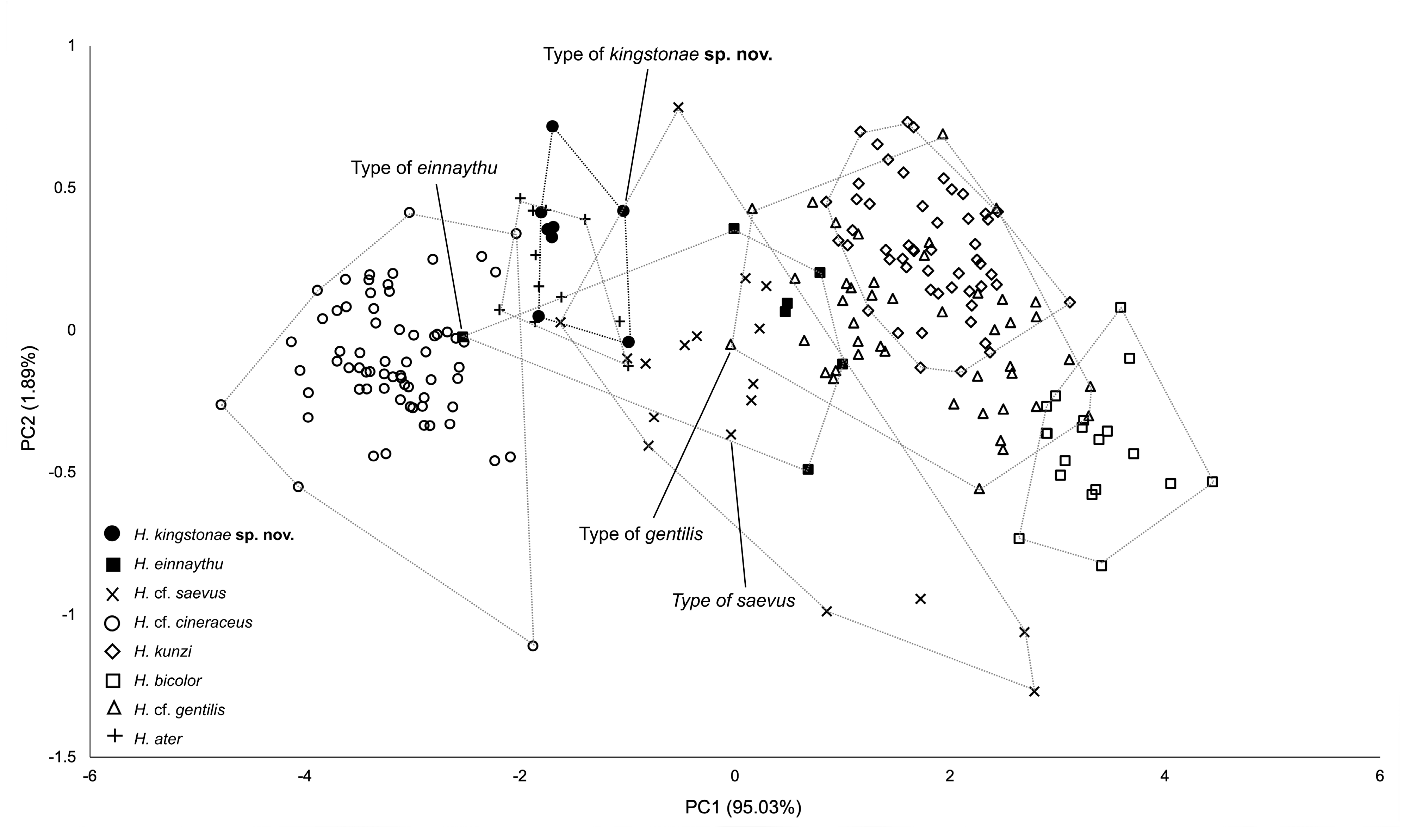
Genetics. The phylogenetic trees based on mitochondrial COI reveal that the new species clusters with H. kunzi and H. bicolor ( Fig. 7 View FIGURE 7 ), with a genetic distance of 2.3% and 4.9%, respectively (see Appendix 3). Although clustered as a subclade of the new species, the Sabah population is only 0.3% different from the specimens from the Thai-Malay Peninsula ( Fig. 7 View FIGURE 7 ; Appendix 3), and no significant difference in morphology were observed. The new species is not closely related to any of other forms currently referred to H. cineraceus . The tree topology based on ND2 and CytB is similar ( Fig. 8 View FIGURE 8 ), as previously noted by Murray et al. (2012; 2108). The ND2 and partial CytB sequences of 4 specimens from the island of Palawan deposited in the FMNH as H. ater ( Esselstyn et al., 2012) belong in the clade of the new species, as sister to the Sabah specimens with less than 1% genetic distance from them. The genetic distances between the new species and H. kunzi and H. bicolor , are 8.0% and 7.3% based on CytB, and 7.3% and 10.5%, respectively, based on ND2. In addition, these two species are clearly different from the new species in terms of morphology (see comparison section below). Morphologically, the new species is similar to H. einnaythu (see comparison section below), which is also present in the region ( Douangboubpha et al., 2011; Douangboubpha, 2019) but has a genetic distance of 9.6% and 10.4% based on COI and ND2, respectively.
In addition, this study provides, for the first time, genetic data (as well as echolocation—see below) of H. einnaythu from Tanintharyi, Myanmar and Thailand. Besides being closely clustered with H. kunzi , H. bicolor and H. kingstonae sp. nov., it also shares a clade with H. cf. gentilis ( Fig. 7 View FIGURE 7 & 8 View FIGURE 8 ).
The samples of H. cf. cineraceus and H. cf. gentilis appeared to be paraphyletic based on mitochondrial COI analyses ( Fig. 7 View FIGURE 7 ), as well as ND2 and CytB ( Fig. 8 View FIGURE 8 ), and suggest multiple cryptic taxa ( Francis et al., 2010; Murray et al., 2012; 2018). However, recent analyses of nuclear markers suggested that they are monophyletic, and the appearance of multiple mtDNA clades may be explained by historical introgression (Yuzefovic et al., 2021). This is in line with our analyses of external, craniodental and bacular morphology that show no clear distinction between different genetic clades within either H. cf. cineraceus or H. cf. gentilis . Although neither the type specimen nor any of H. cineraceus (sensu stricto) from India were examined, the specimens from Bhutan and north Myanmar ( Fig. 1 View FIGURE 1 ) included in this study agreed morphologically with specimens of H. cf. cineraceus from Thailand and elsewhere in SE Asia.
Comparison with similar species. Hipposideros kingstonae sp. nov., with a mean FA of 39.0 mm and GTL 16.29 mm, is intermediate in size between the smaller H. cf. cineraceus (FA 35.3 mm; GTL 15.55 mm) and the larger H. kunzi (FA 43.0 mm; GTL 18.05mm), H. bicolor (FA 46.3 mm; GTL 18.95 mm) and H. cf. gentilis (FA 41.7 mm; GTL 17.82mm) ( Tables 1 View TABLE 1 and 2 View TABLE 2 ). However, it can be readily distinguished from these species by the distinct rounded swollen internarial septum ( Fig. 9a View FIGURE 9 ), which is narrow and parallel-sided in H. cf. cineraceus ( Fig. 9d View FIGURE 9 ), and H. bicolor ( Fig. 9f View FIGURE 9 ), or more or less triangular with a wider base in H. kunzi ( Fig. 9e View FIGURE 9 ). The internarial septum of H. cf. gentilis (not illustrated) is less swollen than that of the new species, and it has a distinctly larger ear (mean 21.0 vs 15.0 mm) (see also figure 4e and 4f in Dounagboubpha et al., 2010). Acoustically, with a frequency of 141.0– 144.0 kHz in the mainland populations and 132.3–138.2 kHz in Borneo, the new species overlaps in FmaxE with H. kunzi (mean 142.5 kHz, min–max 135.1–146.7 kHz) ( Table 1 View TABLE 1 ). However, as mentioned above, they are clearly distinguished by body size and the shape of the internarial septum. As expected, the smaller H. cf. cineraceus has an average higher FmaxE (147.9 kHz in Thailand; 149.0 kHz in individuals caught at Madai Caves, Sabah), while the larger H. bicolor and H. cf. gentilis have a lower FmaxE, 132.0 and 132.8 kHz, respectively ( Table 1 View TABLE 1 ).
Hipposideros kingstonae sp. nov. most closely resembles H. einnaythu , particularly in the shape of the internarial septum ( Fig. 9a View FIGURE 9 vs Fig. 9b View FIGURE 9 ), forearm length (39.0 vs 40.8 mm) and call frequency (139.0 vs 140.3 kHz) ( Table 1 View TABLE 1 ). The skull measurements between the two species also overlap considerably, with H. kingstonae averaging slightly smaller than H. einnaythu in all characters, except the PC that is larger (2.62 vs 2.53 mm, Table 2 View TABLE 2 ). However, H. einnaythu , has one rudimentary lateral leaflet on each side of the noseleaf, although it can be hardly seen in some individuals, and the baculum of H. kingstonae is very small with a distinct bifid tip ( Fig. 4a View FIGURE 4 ) and only about one-third that of H. einnaythu in length (~ 1.5 mm; Fig. 4b View FIGURE 4 ). In addition, as mentioned above, the genetic distance between the two species is 9.6% and 10.4% based on COI and ND2, respectively.
......continued on the next page
The availability of recently collected specimens from Thailand and Myanmar allowed for a more comprehensive comparison of both morphology (including bacula) and genetics between einnaythu and ater (sensu stricto)—which also support the specific distinction of kingstonae . The shape of the internarial septum of ater is triangular, and the baculum is rather straight, ~ 1.7 mm in length, without prongs at the tip (see figure 2a and 4a in Douangboubpha et al., 2011).
As H. ater (sensu stricto) is currently believed to be restricted to India and Sri Lanka ( Douangboubpha et al., 2011), while the taxon antricola from the Philippines is most likely quite distinct ( Simmons and Cirranello, 2022), the identity of specimens from Indonesia and Sabah ( Hill and Francis, 1984) referred to ‘ H. ater ’ and ‘ H. cf. ater ’ is uncertain. The internarial septum of specimens from Java and Sabah ( Fig. 9c View FIGURE 9 ) is similar to that of ater from India but the external and cranial measurements are larger ( Table 1 View TABLE 1 and 2 View TABLE 2 ). It is hereby provisionally assigned to ‘ H. cf. saevus K. Andersen, 1918’. Although a taxonomic revision is needed, nonetheless, it differs from kingstonae in the shape of the internarial septum ( Fig. 9a View FIGURE 9 vs 9c) and in the shape of the skull.
In the skull, kingstonae has well-developed nasal swellings, with a shallow depression behind ( Fig. 5a View FIGURE 5 ). However, the frontal depression of einnaythu is more pronounced, deeper with a well-defined supraorbital ridge ( Fig. 5b View FIGURE 5 ). In contrast, this part is flat or slight curved upward in the specimens of H. cf. saevus ( Fig. 5c View FIGURE 5 ).
The combination of the forearm length (FA) together with call frequency (FmaxE) can be very useful for provisionally identifying kingstonae from other species ( Fig. 10 View FIGURE 10 ). The biplot shows clear groupings, particularly between the new species and smaller species ( H. cf. cineraceus ) and larger species ( H. kunzi , H. bicolor and H. cf. gentilis ) ( Fig. 10 View FIGURE 10 ). The Principal Components Analysis (PCA) based on 14 cranial and dental measurements of 56 specimens also shows the same pattern of groupings ( Fig. 11 View FIGURE 11 ) and the new species can be distinguished from the species overlapped in size ( H. einnaythu , H. ater and H. cf. saevus) by the combination of characters described above.
Ecology, distribution and conservation notes. Hipposideros kingstonae sp. nov. is here documented from five localities, two in the deep south of peninsular Thailand, one in peninsular Malaysia, and two in the Malaysian state of Sabah, on the Borneo island ( Fig. 1 View FIGURE 1 ). The morphology of the noseleaf matches the description of “ H. cineraceus ” in Payne and Francis (1985) suggesting that many previous records from Borneo (including those from Segarong caves and Baturong caves mentioned by Hill and Francis 1984) likely represent the new species. In addition, published DNA sequences from the Philippine island of Palawan are genetically very close to those from Sabah, suggesting that the species is also present on that island, which is so geographically and biogeographically close to Borneo that it was almost connected during the Last Glacial Maximum ( Piper et al., 2011). The type series of the new species is from lowland primary rainforest at an elevation between 22 and 170 m a.s.l. In Sabah, 9 individuals of the species were captured in the understory of lowland rainforest near the large cave system of Madai, at 110 m a.s.l. presumably emerging from the caves along with specimens of H. cf. cineraceus . One specimen was captured inside the lower montane forest on the slopes of Gunung Kinabalu at 1600 m a.s.l., suggesting it is a forest-restricted bat that forages in forest gaps in the understorey or near the edge of the forest. The roosting sites of the species are not known, but several individuals in Sabah were caught near large limestone caves, suggesting it likely roosts in the caves. However, elsewhere, individuals were caught far from known caves and may roost in tree hollows. The current threats to the new species are not known, although loss of habitat due to agriculture, including tree plantations, is a major concern in much of the Thai-Malay Peninsula and Borneo.
| BB |
Buffalo Bill Museum |
| MW |
Museum Wasmann |
| ML |
Musee de Lectoure |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |