Doryctobracon adaimei Marinho and Penteado-Dias, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4353.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:DF8FFBD9-1A85-4EB7-A20C-669D219634B7 |
|
DOI |
https://doi.org/10.5281/zenodo.6039804 |
|
persistent identifier |
https://treatment.plazi.org/id/7E53462B-1C9E-4FEC-A205-4D9877675D4A |
|
taxon LSID |
lsid:zoobank.org:act:7E53462B-1C9E-4FEC-A205-4D9877675D4A |
|
treatment provided by |
Plazi |
|
scientific name |
Doryctobracon adaimei Marinho and Penteado-Dias |
| status |
sp. nov. |
Doryctobracon adaimei Marinho and Penteado-Dias sp. nov.
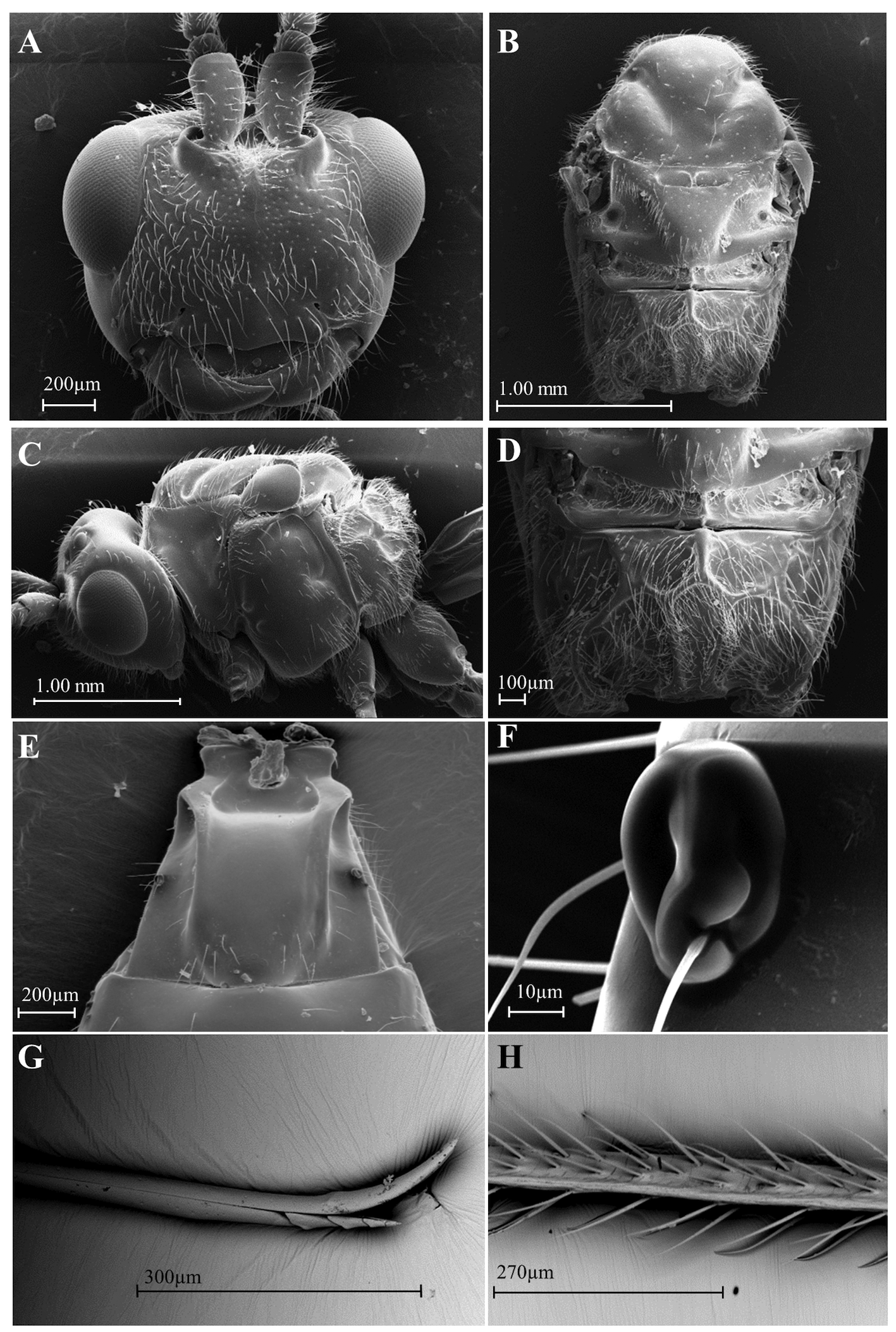
( Figs 1 C–C View FIGURE 1 2, D–D2, E–E2 View FIGURE2 , 3 B View FIGURE 3 , 5 A–H View FIGURE 5 )
http://www.zoobank.org/NomenclaturalActs/7 E53462 View Materials B-1C9E-4FEC-A205-4D9877675D4A
Doryctobracon View in CoL sp. 2: Veloso et al. 1996 (Cerrados de Goiás, GO ex Anastrepha View in CoL spp. in Pouteria gardneriana View in CoL (DC.) Radlk and P. ramiflora Radlk View in CoL ). Bomfim et al. 2007 (Tocantins, TO ex Anastrepha coronilli View in CoL in Bellucia grassularioides ); Braga Filho et al. 2001, Leopoldo Bulhões, GO in Salacia crassifolia (Mart.) Peyr ; Deus et al. 2010 (Pedra Branca do Amapari, AP, ex Anastrepha atrigona View in CoL in Geissospermum argenteum View in CoL ); Silva et al. 2011 (Laranjal do Jari, ex Anastrepha atrigona View in CoL in Geissospermum argenteum View in CoL ; ex A. fraterculus View in CoL in Psidium guajava View in CoL ; Vitória do Jari, AP, ex A. atrigona View in CoL in Geissospermum argenteum View in CoL ; ex A. striata View in CoL in Psidium guajava View in CoL ).
Diagnosi s. Differs from D. areolatus View in CoL in having the fore wing infumate, with a broad rounded hyaline spot from immediately after the stigma to the middle of R1a and not reaching the posterior margin of the fore wing, ending at the middle of the second subdiscal cell (2nd disc) ( Fig 3B View FIGURE 3 ). Stigma dark brown ( Fig 3B View FIGURE 3 ). In D. whartoni sp. nov. the fore wing has a hyaline spot in the anal area and a hyaline stripe in the central portion, and the stigma is yellow ( Fig 3A View FIGURE 3 ). Hind legs with chromatic variations (orange, orange-brown, dark brown to black) on the entire leg or on part of it ( Fig 1C View FIGURE 1 2, D2, E2 View FIGURE2 ). Tegula yellowish orange as in D. whartoni sp. nov. (dark brown in D. areolatus View in CoL ). Propodeum with one short basal transverse keel projecting laterally in the anterior portion of the areola reaching the longitudinal lateral keel, as in D. whartoni sp. nov. ( Fig 5D View FIGURE 5 ). Ovipositor apex similar to D. areolatus View in CoL , with a dorsal node and ventral serrations ( Fig 5G View FIGURE 5 ). Ventral serrations morphologically similar to those of D. areolatus View in CoL . In D. whartoni sp. nov., the serrations are more conspicuous and distinct ( Fig 4E View FIGURE 4 ).
Description Female. Length of body, excluding ovipositor 5.5–6.3 mm.
Head. 1.4–1.2× wider than long; 1.5–1.3× wider than width of mesoscutum. Face polished, bright, and distinctly setose; midridge smoother, restricted between toruli ( Fig 5A View FIGURE 5 ); distance between toruli equal to distance from toruli to eye ( Fig 5A View FIGURE 5 ). Antenna longer than body, 8.4–8.7 mm in length, with 53 to 55 flagellomeres, first flagellomeres about 1.1–1.2× longer than second; 1.5–2.1× longer than wide. Eye large, 1.2–1.3× wider than high ( Fig 5C View FIGURE 5 ); in dorsal view, eye 1.6–2.0× wider than temples; malar space 0.4–0.5× longer than height of eyes. Clypeus 2.6–3.9× wider than high, polished, slightly convex and lateral margin slightly curved with sparse setae two to three times longer than setae on face ( Fig 5A View FIGURE 5 ); clypeus sinuate, similar to D. whartoni sp. nov., protruding as lobe medially on ventral margin; labrum partially covered by clypeus; distinct opening between clypeus and mandibles ( Fig 5A View FIGURE 5 ).
Mesosoma. 1.2–1.5× longer than high; 1.7–2.0× longer than wide; 1.3–1.5× higher than wide; pronotum not visible dorsally; median lobe of mesoscutum and lateral lobes as in D. whartoni sp. nov., polished, shiny with few sparse setae; margins of lateral lobes with few setae and shorter than D. whartoni sp. nov. ( Fig 5B View FIGURE 5 ); notaulus smooth, complete, deeper anterior to margin of mesoscutum and later shallower, reunited in broad and polished impression without midpit ( Fig 5B View FIGURE 5 ); scutellar groove divided into two large pits by median longitudinal septum; scutellum smooth with few or weak punctures, few setae on margins, robust or wide in apical portion, similar to D. areolatus ( Fig 5B View FIGURE 5 ); mesopleuron smooth; propodeum setose with median anterior basal keel (0.11–0.15) and complete posterior areola. As in D. whartoni sp. nov., laterally, in the anterior region of the areola, one short basal transverse keel extends to reach the lateral longitudinal keel, which is prominent and distinctly curved, from the posterior half of the propodeum ( Fig 5D View FIGURE 5 ).
Wings. Fore wing 5.3–6.3 mm length; stigma wide, 3.0–4.2× longer than wide, with vein r slightly projecting from midpoint; (RS+M)a slightly sinuate posteriorly, 1.0–1.6× longer than 3RSa; 2RS 0.7–1.3× longer than 3RSa, 1.2–1.5× longer than 1m-cu and 1.6–2.6× longer than r-m; 3RSa 1.9–3.0× longer than r; 3RSb ending almost at wing tip; 2M 1.6–1.9× longer than 3RSa; (RS+M)b absent; 1cu-a straight line separated from 1M by 0.17–0.30 in length. Fore wing with a rounded hyaline spot that begins from immediately after stigma and ends in the middle of R1a, and before the wing posterior margin at second subdiscal cell (2nd disc). Length of hind wing 3.4–5.0 mm with m-cu, curved, distinctly pigmented just beyond the half toward to the wing margin.
Metasoma. 1.2–2.3× longer than wide and 1.0–2.3× wider than high; T1 length 0.9–1.0 mm greater than apex width; metasoma not sculptured, smooth and bright; T1 apex about 1.2–1.4× width of base; T1 with two dorsal keels developed at the base that gradually decrease from the posterior half, becoming weak or indistinct ( Fig 5E View FIGURE 5 ); spiracles in half of T1 modified in two oval structures that protrude from the smooth surface, with or without setae ( Fig 5E, F View FIGURE 5 ); ovipositor about 5.5–6.3 mm long; ovipositor tip with a dorsal node and ventral serrations that are less developed than in D. whartoni sp. nov. ( Fig 5G View FIGURE 5 ); ovipositor sheath twice metasoma length, with 4–5 rows of setae ( Fig 5H View FIGURE 5 ).
General coloration. Yellowish orange; first and second pair of legs bright yellow, third pair quite variable, may have all segments with blackish or dark-brown spots ( Fig 1C View FIGURE 1 2 View FIGURE2 ); tibiae and tarsi brown ( Fig 1D View FIGURE 1 2 View FIGURE2 ) or coxa, trochanters, and femur yellow ( Fig 1E View FIGURE 1 2 View FIGURE2 ); tegula yellowish orange; ovipositor sheath and antenna dark brown; apices of mandibles black; T2, T3, and T 4 may have black stripes; wings infumate; fore wing with broad rounded hyaline spot that begins from immediately after stigma to middle of R1a and in middle of second subdiscal cell (2nd disc). Veins and setae yellow in the hyaline spot; stigma, veins, and bristles dark brown in infumate area ( Fig 3B View FIGURE 3 ).
Male. Similar to females, but usually last tergite with dark-brown or black spots. Head in dorsal view 1.7× wider than mesoscutum width, 2.0× wider than face; in dorsal view, eye 1.85× wider than temple; face 1.5–1.7× wider than high; malar space 0.42–0.44× height of eyes; clypeus 2.9–3.2× wider than high; antennae with 50 to 51 flagellomeres; first flagellomere 1.1-1.2× longer than second, 1.8–1.9× longer than wide; mesosoma 1.9× longer than wide, 1.3–1.4× higher than wide; metasoma 2.4–2.7× longer than wide, 1.1–1.4 wider than high.
Type material. Holotype. Female (DCBU 270198), BRAZIL: Amapá, Serra do Navio, 00°55’57.0”N, and 051°55’14.8”W 01.ii.2006, reared from fruit fly larva in fruits of guava ( Psidium guajava ), collr. R. A. Silva. Paratypes, with same data as holotype, 2 females (DCBU 270199, DCBU 270200), 1 male (DCBU 270201), collr. R. A. Silva and 1 female (ESALQ); 1 male, Tocantins, 28.ii.2005, reared from fruit fly larva Anastrepha coronilli in “Goiaba-de-anta” ( Bellucia grossularioides L.), collr. M. A. Uchôa-Fernandes; 2 females, 1 male, Goiás, Leopoldo Bulhões, 14. ix. 1999, reared from fruit fly larva Anastrepha spp. In “bacupari” ( Salacia crassifolia ) collr. V.R.S Veloso, (ESALQ).
Etymology. This species is named after Ricardo Adaime, who has provided valuable information of many Anastrepha species, their host fruits, and parasitoids in Amapá, Brazil.
GenBank accession numbers. Doryctobracon adaimei sp. nov. Amapá, FJ560535 View Materials (ITS2) ; Tocantins, FJ560536 View Materials (ITS2) ; Goiás, FJ560537 View Materials (ITS2) ; Amapá, FJ560543 View Materials (28SD2) ; Tocantins, FJ560544 View Materials (28SD2) ; Goiás, FJ560545 View Materials (28SD2).
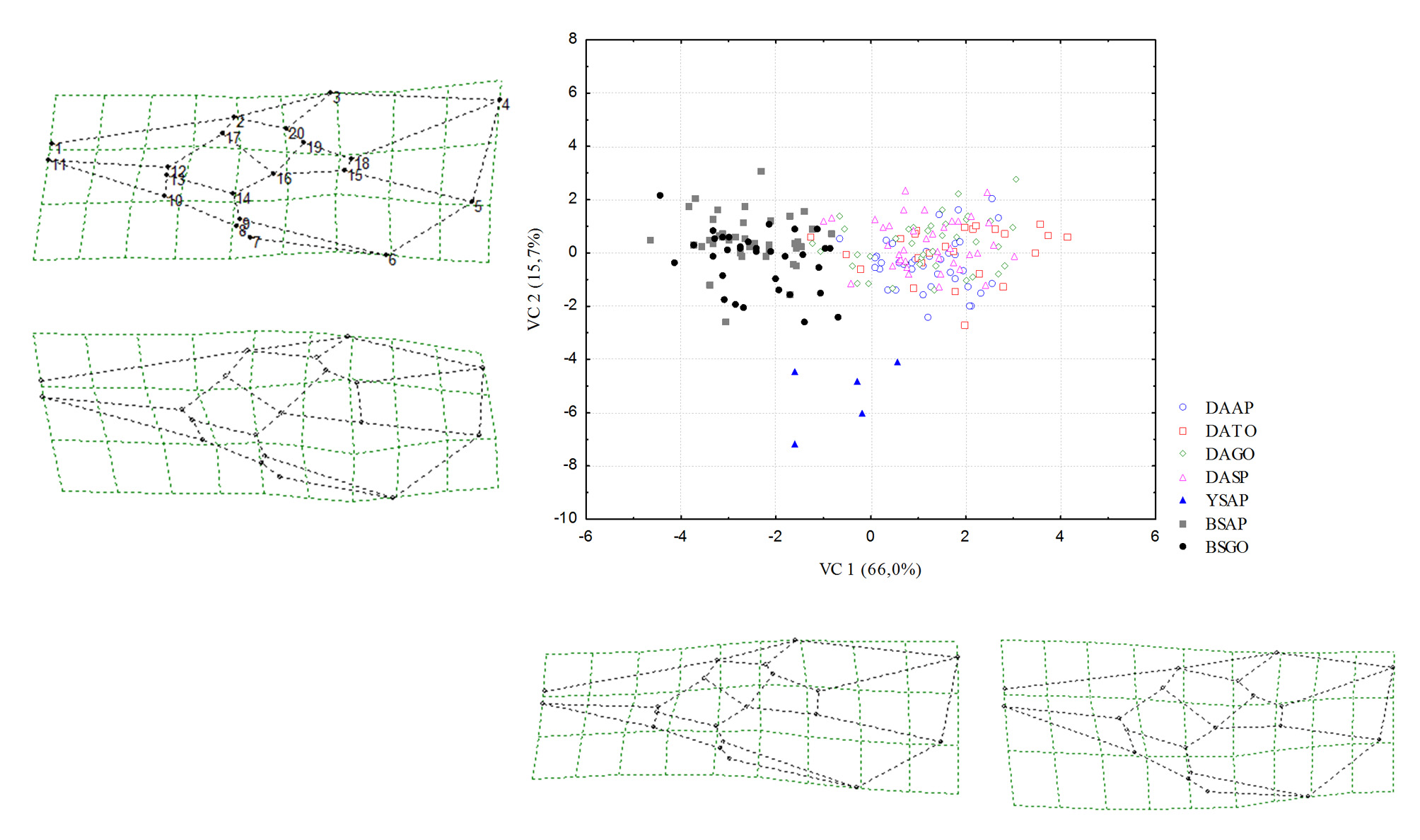
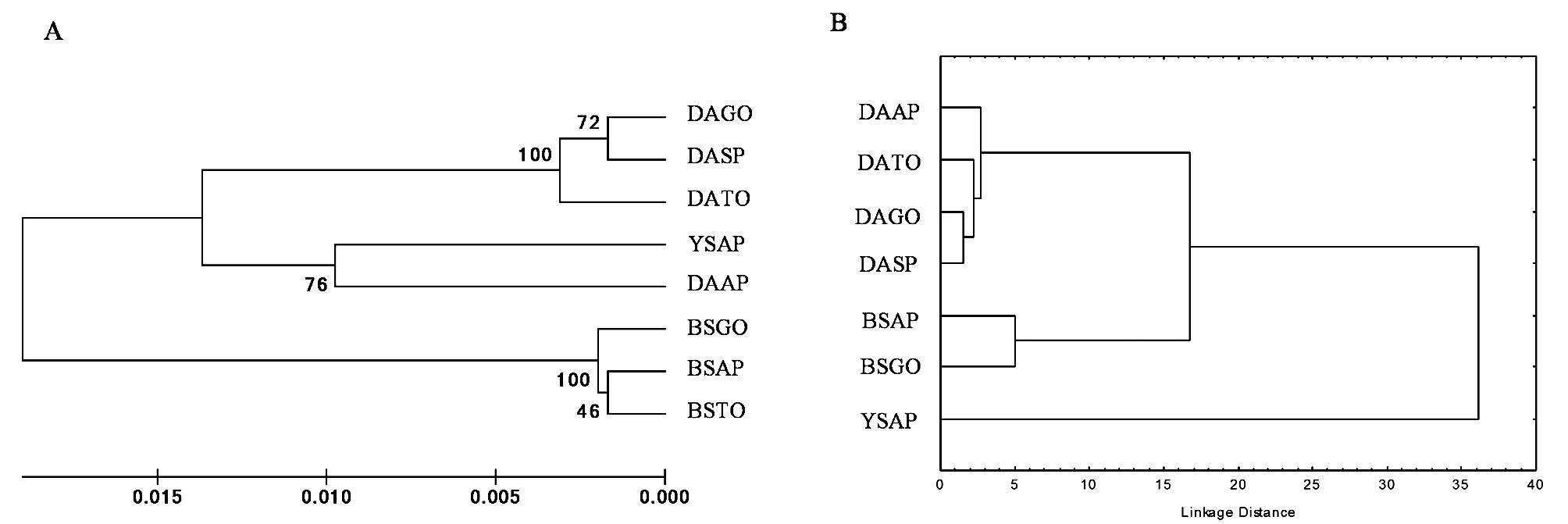
Morphometry. Geometric morphometry generated 36 relative deformation measurements (k=2n–4), where k represents the total number of relative deformations, and n the number of anatomical markers (20). The results of the multivariate analysis (CVA) of populations of male and female D. areolatus , D. whartoni sp. nov. and D. adaimei sp. nov. were statistically significant, Wilks’ Lambda (p <0.0001), Pillai’s Trace (p <0.0001), Hotelling- Lawley Trace (p <0.0001), and Roy’s Greatest Root (p <0.0001). The first two canonical axes explained 66.0% and 15.7% of the data variability, respectively. In the scatter plot, we observed the complete separation of the population groups in the space of canonical variables CV1 and CV2 ( Fig 6 View FIGURE 6 ). Specimens termed D. whartoni sp. nov., collected only in the state of Amapá, formed an isolated group of D. adaimei sp. nov. and of D. areolatus . Doryctobracon adaimei sp. nov. separated completely from the groups formed by the other species and revealed total overlap of their populations collected in the states of Goiás and Amapá; among the populations of D. areolatus , there was also the formation of distinct grouping with D. whartoni sp. nov. and D. adaimei sp. nov., and total overlap of their populations in the states of Amapá, Tocantins, Goiás and São Paulo ( Fig 6 View FIGURE 6 ). The Mahalanobis distance matrix revealed a lower degree of morphological similarity for D. whartoni sp. nov. in relation to the distinct populations of D. areolatus and D. adaimei sp. nov. We also observed the same result for population of D. adaimei sp. nov. in relation to the populations of D. areolatus ; however, the Mahalanobis distances between samples of D. whartoni sp. nov. and D. adaimei sp. nov. were fairly wide, i.e. D. whartoni sp. nov. (AP) - D. adaimei sp. nov. (AP) = 38.39%, and D. whartoni sp. nov. (AP) - D. adaimei sp. nov. (GO) = 34.96% ( Fig 7 View FIGURE 7 , Table 2).
Molecular markers. We detected intraspecific size variation for both molecular markers tested when comparing populations of D. areolatus from different geographical regions. The size variation was larger for the ITS2, which ranged from 564 bp for D. areolatus from the state of Amapá to 590 bp for specimens from the state of São Paulo. ITS2 was 587 bp long in samples from the states of Goiás and Tocantins. The size of 28S-D2 from samples of D. areolatus differed by no more than 5 bp in length, ranging from 381 bp for specimens from Amapá to 386 bp for those from Goiás. Specimens from Tocantins and São Paulo had intermediate sizes (384 and 385 bp, respectively). Despite the variation in size, these markers shared very high sequence similarities ( Table 3). However, no size variation was observed for both markers for the species identified as D. whartoni sp. nov. and D. adaimei sp. nov. ITS2 for D. whartoni sp. nov. from Amapá was 585 bp long, while ITS2 for D. adaimei sp. nov. from Amapá, Tocantins, and Goiás was 567 bp in length. The 28S-D2 of D. whartoni sp. nov. from Amapá was 385 bp long, and 386 (Amapá and Tocantins) and 387 bp long (Goiás) for specimens of D. adaimei sp. nov. Pairwise sequence similarities of ITS2 and 28S-D2 among D. whartoni sp. nov., D. adaimei sp. nov., and D. areolatus were very low ( Table 3).
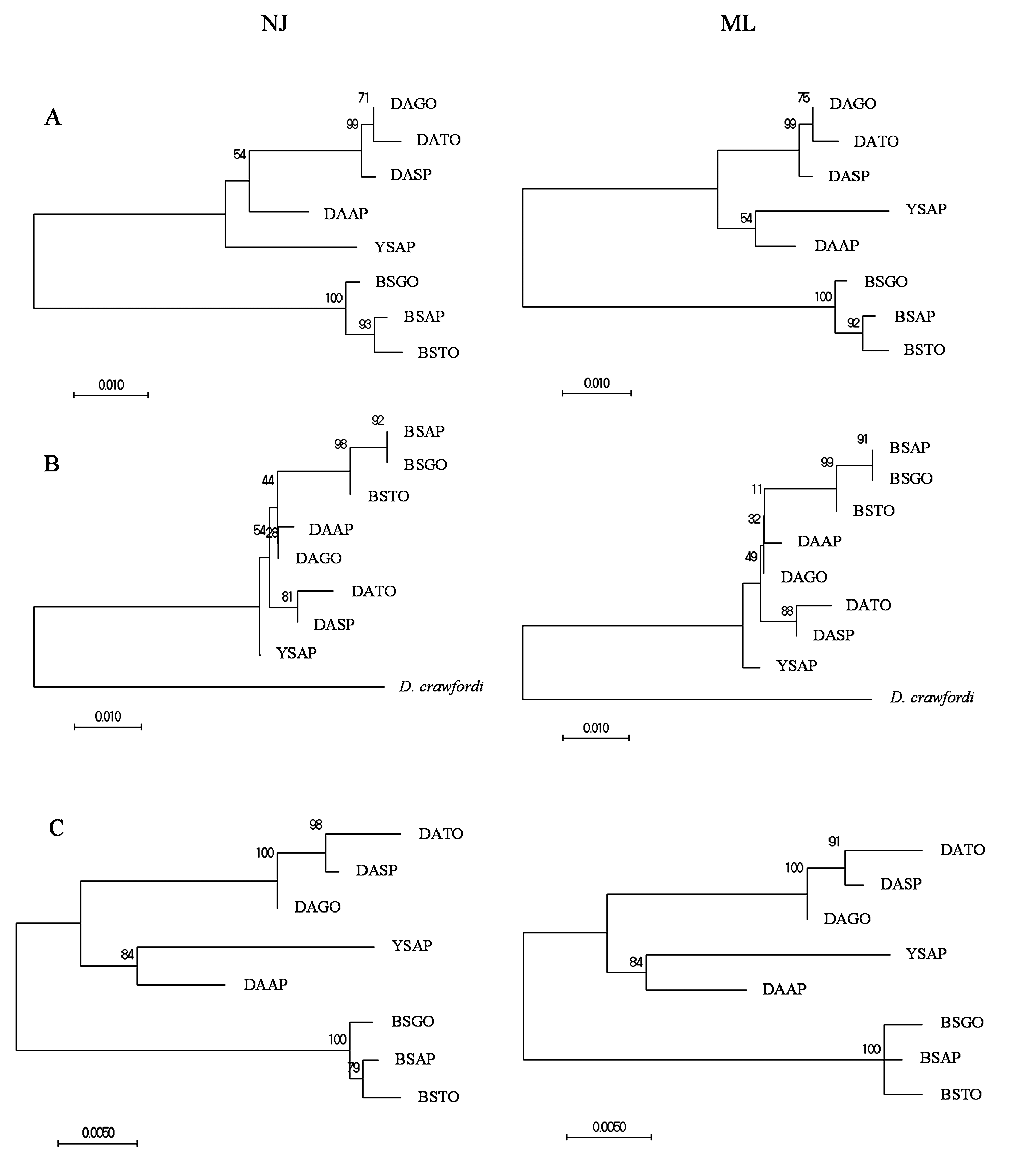
Phylogenetic reconstruction of specimens of Doryctobracon species based on the isolated and concatenated sequences of the molecular markers ITS2 and 28S-D2, using maximum likelihood (ML) and neighbor-joining (NJ) methods, produced trees sharing similar topologies and well-defined clades ( Fig 8 View FIGURE 8 ). In all cases, molecular analysis consistently indicated variation in D. areolatus from different collection sites (Amapá, Goiás, Tocantins, and São Paulo), with specimens from Amapá being the most divergent. The most variation in clade definition between the ML and NJ methods was found for the positioning of D. areolatus from Amapá and D. whartoni sp. nov. ( Fig 8 View FIGURE 8 ). Nevertheless, the topologies produced with the concatenated sequences left no doubts regarding the external grouping of specimens of D. areolatus from Amapá from a more internal clade of D. areolatus from the other states. In the concatenated analysis, D. whartoni sp. nov. resolved in a subclade with the divergent D. areolatus from Amapá, but was positioned on a long branch away from D. areolatus ( Fig 8C View FIGURE 8 ). The remaining samples of D. areolatus grouped together in a more internal subclade ( Fig 8 View FIGURE 8 ). Samples of D. adaimei sp. nov. always resolved in a defined clade, in the analysis with either the isolated or concatenated molecular markers. In the analysis with the concatenated sequences, the clade of D. adaimei sp. nov. was the first to branch out from all of the remaining samples. The NJ analysis resolved specimens belonging to D. adaimei sp. nov. from Amapá and Tocantins in a more internal subclade from specimens from Goiás, while ML placed specimens from all three populations in a single clade separated from each other by short branches ( Fig 8 View FIGURE 8 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Doryctobracon adaimei Marinho and Penteado-Dias
| Marinho, Cláudia F., Cônsoli, Fernando L., Penteado-Dias, Angélica Maria & Zucchi, Roberto A. 2017 |
D. whartoni
| Marinho & Cônsoli & Penteado-Dias & Zucchi 2017 |
D. whartoni
| Marinho & Cônsoli & Penteado-Dias & Zucchi 2017 |
D. whartoni
| Marinho & Cônsoli & Penteado-Dias & Zucchi 2017 |
D. whartoni
| Marinho & Cônsoli & Penteado-Dias & Zucchi 2017 |
Anastrepha coronilli
| Carrejo & Gonzalez 1993 |
Geissospermum argenteum
| R. E. Woodson 1939 |
Geissospermum argenteum
| R. E. Woodson 1939 |
Geissospermum argenteum
| R. E. Woodson 1939 |
Doryctobracon
| Enderlein 1920 |
Anastrepha atrigona
| Hendel 1914 |
Anastrepha atrigona
| Hendel 1914 |
A. atrigona
| Hendel 1914 |
Anastrepha
| Schiner 1868 |
A. striata
| Schiner 1868 |