Culicoides (Monoculicoides) occidentalis Wirth and Jones
|
publication ID |
https://doi.org/ 10.5281/zenodo.6391684 |
|
publication LSID |
lsid:zoobank.org:pub:CBD29188-143B-44DF-BE21-1654D50D8621 |
|
persistent identifier |
https://treatment.plazi.org/id/E8511E53-FF94-EF35-6A8A-FEA0FE24F862 |
|
treatment provided by |
Felipe |
|
scientific name |
Culicoides (Monoculicoides) occidentalis Wirth and Jones |
| status |
|
Culicoides (Monoculicoides) occidentalis Wirth and Jones View in CoL
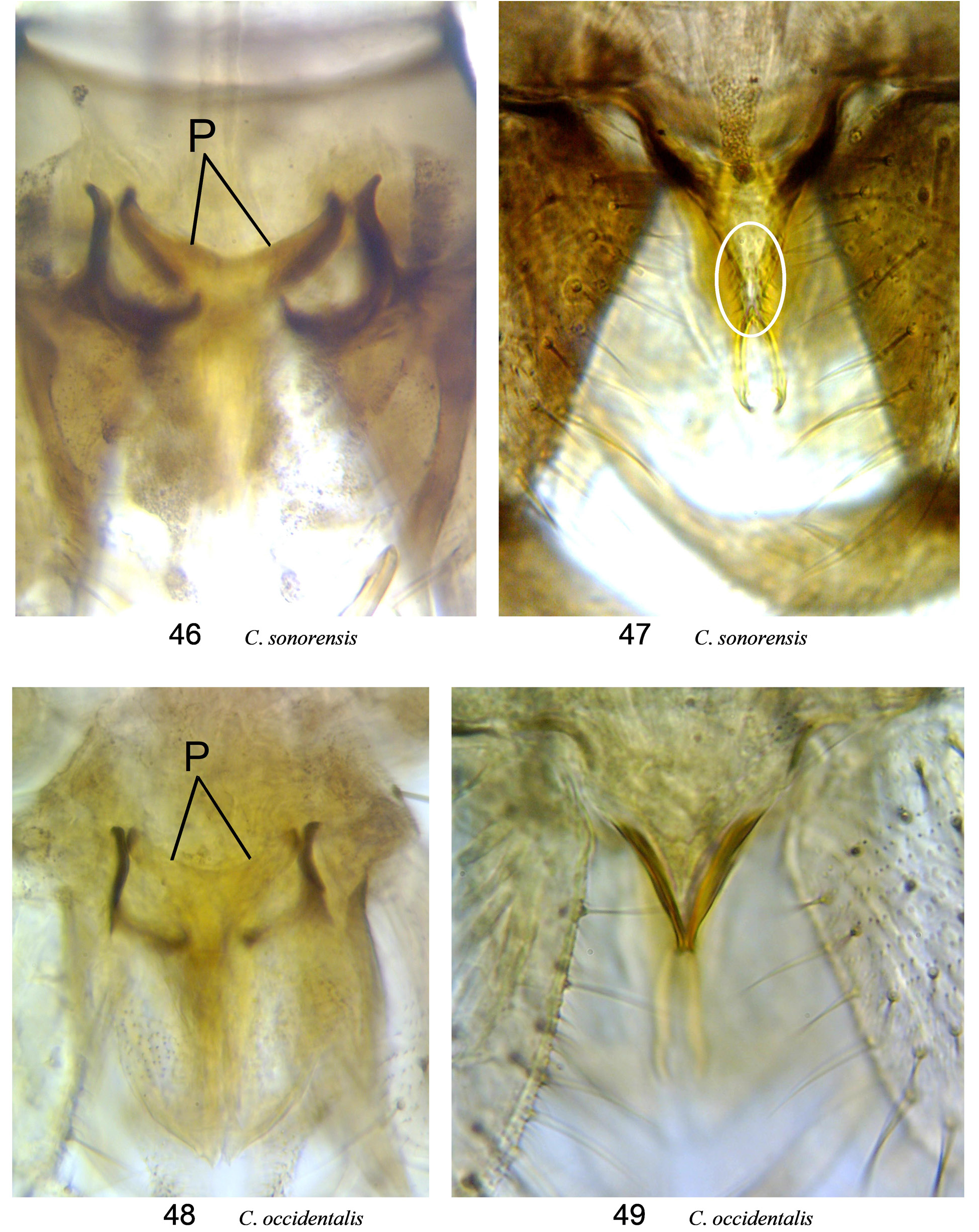
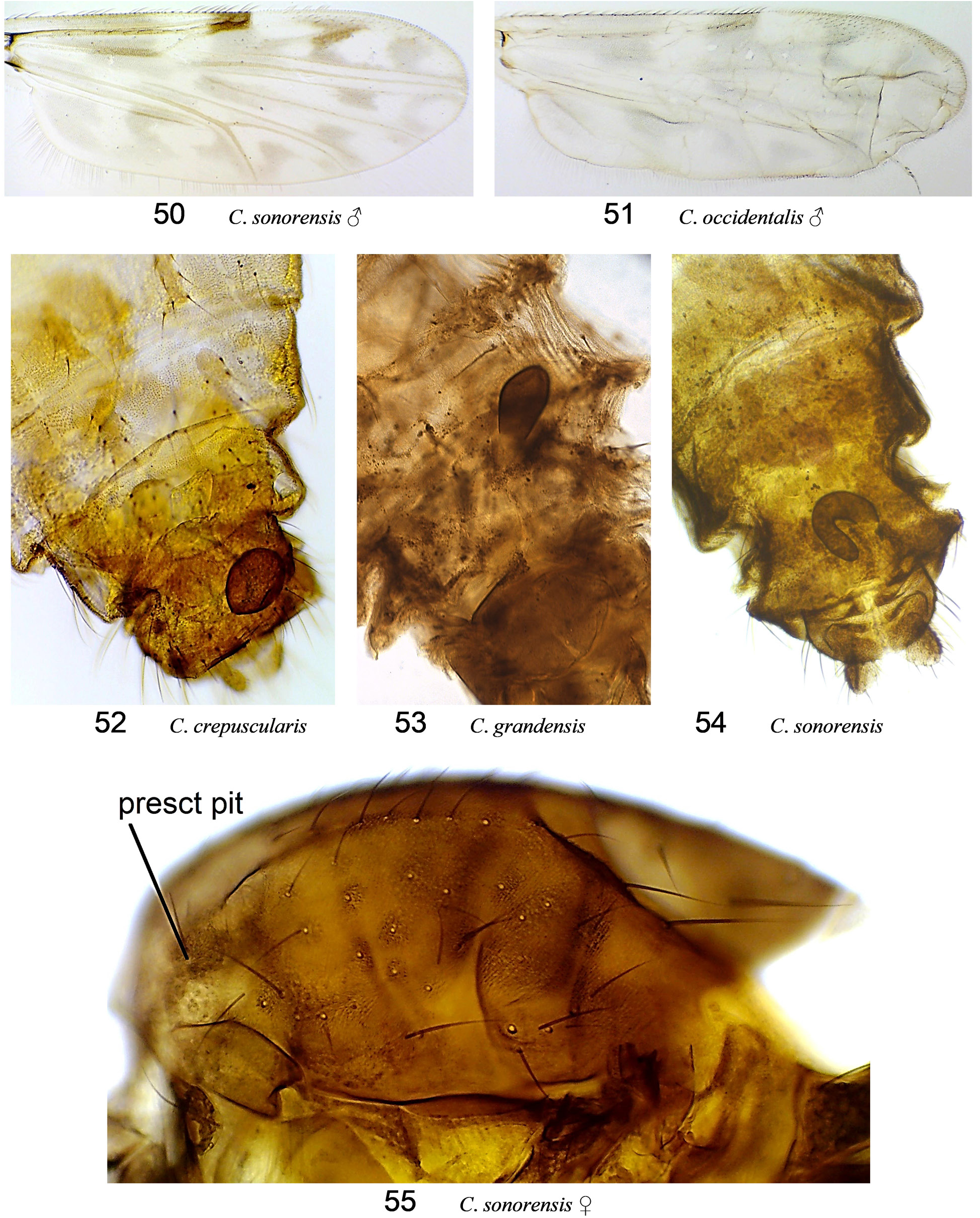
( Fig. 48, 49 View Figures 46–49 , 51 View Figures 50–55. 50 )
Culicoides (Monoculicoides) variipennis occidentalis Wirth and Jones, 1957: 21 View in CoL (diagnosis; fig. female palpus, mesonotum, wing; California). Jorgensen 1969: 27 (in part; key; quantitative characters; female; male genitalia; seasonal distribution; fig. male genitalia, parameres, female wing, spermatheca, palpus, antenna; Washington). Wirth et al. 1985: 30 (numerical characters). Wirth et al. 1988: 56 (numerical characters; fig. female wing). Tabachnick 1992 (genetic comparison of C. v. sonorensis View in CoL , C. v. occidentalis View in CoL , and C. v. variipennis View in CoL ). Velten and Mullens 1997 (comparison of C. v. sonorensis View in CoL and C. v. occidentalis View in CoL ; fig. pupal terminalia, male aedeagi; biology).
Culicoides (Monoculicoides) occidentalis: Jorgensen 1969: 32 View in CoL (status change). Downes 1978: 63 (status change as C. o. occidentalis View in CoL ; fig. female palpus). Holbrook et al. 2000: 70 (key; diagnosis; fig. female palpus, aedeagus). Borkent and Spinelli 2000: 36 (in Neotropical catalog). Shults and Borkent 2018: 459 View Cited Treatment (key; numerical characters; male pupa, female pupa; fig. habitus, respiratory organ, female dorsal apotome, mouthparts, thoracic setae, abdominal segments 8–9, male abdominal segment 4).
Culicoides variipennis (Coquillett) View in CoL , misidentified in part: Wirth 1952a: 180, 252 (key; female; male genitalia; pupa, larva; distribution; fig. female wing, dorsal thoracic patterns, palpus, pupa, larva). Bullock 1952: 18 (key; female; male genitalia; biology; habitat and biotic associations; fig. larvae, pupae; seasonal distribution; Utah: Salt Lake County). Rees and Bullock 1954 (Utah: Salt Lake County). Foote and Pratt 1954: 34 (key; diagnoses of female, male, pupa; biology; fig. female wing, mesonotum, palpus, male genitalia). Fox 1955: 258 (key and diagnoses of subgenera; species key; taxonomy). Hensleigh and Atchley 1977: 379 (morphometric analysis). Blanton and Wirth 1979: 161 (key; numerical characters; female; male genitalia; pupa, larva; fig. female antenna, palpus, wing, eye separation, spermatheca, leg, male genitalia, parameres; larval habitat; feeding habits; seasonal distribution). Wirth and Morris 1985: 165 (reevaluation of C. variipennis View in CoL complex). Murphree and Mullen 1991: 354 (Borax Lake, California; key; larva; numerical characters; fig. head, thorax, epipharynx, hypostoma, mandible, caudal segment).
Culicoides variipennis australis Wirth and Jones View in CoL , misidentified: Jones 1961b: 703 (in part; pupal habitats; Utah). Atchley 1967: 975 (synonym subordinate to C. v. sonorensis View in CoL , in part; New Mexico).
Culicoides variipennis sonorensis Wirth and Jones View in CoL , misidentified: Atchley 1967: 974 (in part; key; numerical characters; female; male genitalia; variation; feeding habits; fig. female wing, palpus, tibial comb, spermatheca, male genitalia, parameres; New Mexico).
Diagnosis. ( Tables 14, 15). (Females morphologically indistinguishable from C. sonorensis .) Dark brown; wing with prominent pattern; in r 3, m 1, m 2, cua 1 extensive and more of dark irregular curves, pale streaks, and zigzags than ovoid spots; scutum with dark spots at seta bases (as in Fig. 55 C View Figures 50–55. 50 . sonorensis); legs with distinct pale banding; female palpal segment 3 swollen 1.8–3.0× longer than wide, with medium to large rounded or irregularly shaped and often partly divided sensory pit; one sclerotized spermatheca, U-shaped, with opening>0.5 the diameter of the spermatheca, without neck (as in Fig. 54 C View Figures 50–55. 50 . sonorensis); parameres fused basally; ventral surface of aedeagus bare, apex deeply bifurcated into bladelike tips.
Distribution. British Columbia, Alberta, south through Washington, Oregon, California, Nevada, Utah (Grand, Salt Lake counties, new state record), Arizona (new state record [ Monarch 2021]), to Baja California, Baja California Sur, with disjunct populations in New Mexico, Texas, Puebla ( Huerta et al. 2012).
Biology. Because of the complex synonymy of the Variipennis group, C. occidentalis , C. sonorensis , and C. variipennis conflate in many records before 2000. Though I have tried to present only data associated with C. occidentalis , it is possible some of it refers to C. sonorensis , C. variipennis , or a combination.
Larval ecology. Culicoides occidentalis is likely to be widespread in highly alkaline or saline sites in Utah. Bullock (1952) collected and reared immatures likely to be C. occidentalis (as C. variipennis ) from a brine (21% salts) borrow pit in Salt Lake County. Jones (1961b) also collected pupae likely to be C. occidentalis (as C. variipennis ) from a sunlit sandy seep area along the margin of a highly saline-water pool near the Great Salt Lake in Salt Lake County; and pupae likely to be both C. occidentalis and C. sonorensis (as C. variipennis australis ) from the nonvegetated sunlit margin of an alkaline stream near Cisco (47 km north-northeast of Moab), Grand County, along with immatures of a Stonei group species (as C. stonei ), C. jamesi , C. haematopotus (may be C. defoliarti ), C. grandensis (as “n. sp.”), and C. crepuscularis . I was able to rear C. occidentalis along with C. sonorensis , C. crepuscularis , and C. mortivallis from mud collected on 10 September 2020 from nonvegetated sunlit alkaline pools in a stream bed in Grand County at 38.96339°N 109.33585°W and 1315 m elevation in the same wash as Jones’s Cisco pupae collection site. Further confirmation of the presence of C. occidentalis and C. sonorensis at that site and C. occidentalis in Salt Lake County was from CO1 gene analysis of specimens collected and tested by Phillip Shults (personal communication).
Rowley (1967) collected or reared immatures from highly alkaline potholes of pH 9.9 in Washington. Colwell (1981) collected larvae in Northern California from near the shoreline of Borax Lake (38.985°N 122.673°W), a highly alkaline pond with conductivity of 3–130 mS/cm, pH 9.2–10, 1000–5000 ppm alkalinity, and 200– 900 ppm boron. He found eggs laid in the eulittoral zone or on floating algae and collected adults near the site February to November. Schmidtmann et al. (2000) reported aquatic habitats of C. occidentalis immatures had significantly higher conductivity, boron, potassium, and chloride, but lower organic matter and significantly lower phosphate, than C. sonorensis habitats. Among these variables, the most significant discriminator was electrical conductivity, which averaged ~9× higher in the C. occidentalis habitats, which included desert lake margins, sloughs at a dry lake edge, a shallow desert pond, a salt marsh, a saline creek, and a saline hot spring ( Schmidtmann 2006).
Adult behavior. Tempelis and Nelson (1971) identified 325 blood meals from mixed C. sonorensis and C. occidentalis in Kern County, California, as: 51% Bovidae (cattle and sheep), 46% Leporidae (rabbits and hares), 1% Canidae (dogs), 1% Equidae (horses), and 1% unidentified mammals. Culicoides occidentalis collected in Northern California had blood meals from black-tailed deer ( Odocoileus hemionus Rafinesque , Cervidae ), black-tailed jackrabbit ( Lepus californicus ), cow ( Bos taurus ), dog ( Canis lupus Linnaeus , Canidae ), goat ( Capra aegagrus ), sheep ( Ovis aries ), donkey ( Equus africanus Linnaeus , Equidae ), horse ( Equus ferus ), pig ( Sus scrofa Linnaeus , Suidae ), and emu ( Dromaius novaehollandiae [Latham], Casuariidae ) ( Hopken et al. 2017). They proposed the aberrant bird (emu) record may be due to the similarity of the emu’s size and CO 2 output to C. occidentalis ’s normal mammalian hosts; and, Koch and Axtell’s (1979) study of Culicoides host attraction support this possibility. It can also be a severe biting pest of humans ( Colwell 1981).
Nelson (1965) and Nelson and Bellamy (1971) collected many males (as C. v. occidentalis ) with CO 2 -baited traps, suggesting the males seek hosts to find females for mating. In addition, C. occidentalis male swarms have been observed at ~ 2 m above the ground, above and downwind of bushes ( Holbrook et al. 2000).
Vector potential. Culicoides occidentalis has not been implicated in bluetongue virus (BTV) or other arbovirus epizootics, and its saline larval habitats tend to be separate from the normal habitats of bovid hosts. In addition, laboratory studies have shown it to have a significantly lower susceptibility to oral infection by BTV than C. sonorensis ( Holbrook and Tabachnick 1995) . However, Hopken et al. (2017) found C. occidentalis more abundant and C. sonorensis less abundant than previously thought in Lake County, California, which has had BTV and epizootic hemorrhagic disease virus (EHDV) activity ( Roug et al. 2012), suggesting C. occidentalis should be studied further as a potential vector.
Symbionts. Mullens et al. (1997b) experimented with the potential biocontrol parasitic nematode, Heleidomermis magnapapula in the laboratory and found it readily entered, infected, developed, emerged from, and killed C. occidentalis larvae. Also, Mullens et al. (2008) reported that others have found C. occidentalis larvae naturally parasitized by nematodes (likely H. magnapapula ) in mud having 6–20 g /L salt content in Virginia.
Remarks. Tabachnick (1992) provided genetic evidence that C. v. variipennis , C. v. sonorensis , and C. v. occidentalis are distinct over their wide geographic ranges and are likely different species. In addition, Velten and Mullens (1997) were able to hybridize C. v. occidentalis and C. v. sonorensis in the laboratory and produce fertile hybrids, but summarized information showing no gene flow between the species in nature, concluding the subspecies are distinct but closely related. Based on this and more genetic evidence, Holbrook et al. (2000) elevated C. variipennis , C. sonorensis , and C. occidentalis to species status; and Hopken (2016) and Hopken et al. (2017) reported CO1 gene evidence confirming C. occidentalis to be distinct from both C. sonorensis and C. variipennis . See C. sonorensis remarks.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Culicoides (Monoculicoides) occidentalis Wirth and Jones
| Phillips, Robert A. 2022 |
Culicoides (Monoculicoides) occidentalis: Jorgensen 1969: 32
| Shults P & Borkent A. 2018: 459 |
| Holbrook FR & Tabachnick WJ & Schmidtmann ET & McKinnon CN & Bobian RJ & Grogan WL 2000: 70 |
| Borkent A & Spinelli GR 2000: 36 |
| Downes JA 1978: 63 |
| Jorgensen NM 1969: 32 |
Culicoides variipennis australis
| Atchley WR 1967: 975 |
| Jones RH 1961: 703 |
Culicoides (Monoculicoides) variipennis occidentalis
| Wirth WW & Dyce AL & Spinelli GR 1988: 56 |
| Wirth WW & Dyce AL & Peterson BV & Roper I. 1985: 30 |
| Jorgensen NM 1969: 27 |
| Wirth WW & Jones RH 1957: 21 |
Culicoides variipennis (Coquillett)
| Murphree CS & Mullen GR 1991: 354 |
| Wirth WW & Morris C. 1985: 165 |
| Blanton FS & Wirth WW 1979: 161 |
| Hensleigh DA & Atchley WR 1977: 379 |
| Fox I. 1955: 258 |
| Foote RH & Pratt HD 1954: 34 |
| Wirth WW 1952: 180 |
| Bullock HR 1952: 18 |