Euscorpius thracicus, Kovařík & Lowe & Byronová & Šťáhlavský, 2020
|
publication ID |
https://doi.org/ 10.5281/zenodo.4648924 |
|
publication LSID |
lsid:zoobank.org:pub:3E4FA71B-1C3A-4BBD-A928-EF5A8344B419 |
|
DOI |
https://doi.org/10.5281/zenodo.4773674 |
|
persistent identifier |
https://treatment.plazi.org/id/E91C87A3-DD3B-E305-1F1C-437C0352FDFE |
|
treatment provided by |
Carolina |
|
scientific name |
Euscorpius thracicus |
| status |
sp. nov. |
Euscorpius thracicus View in CoL sp. n.
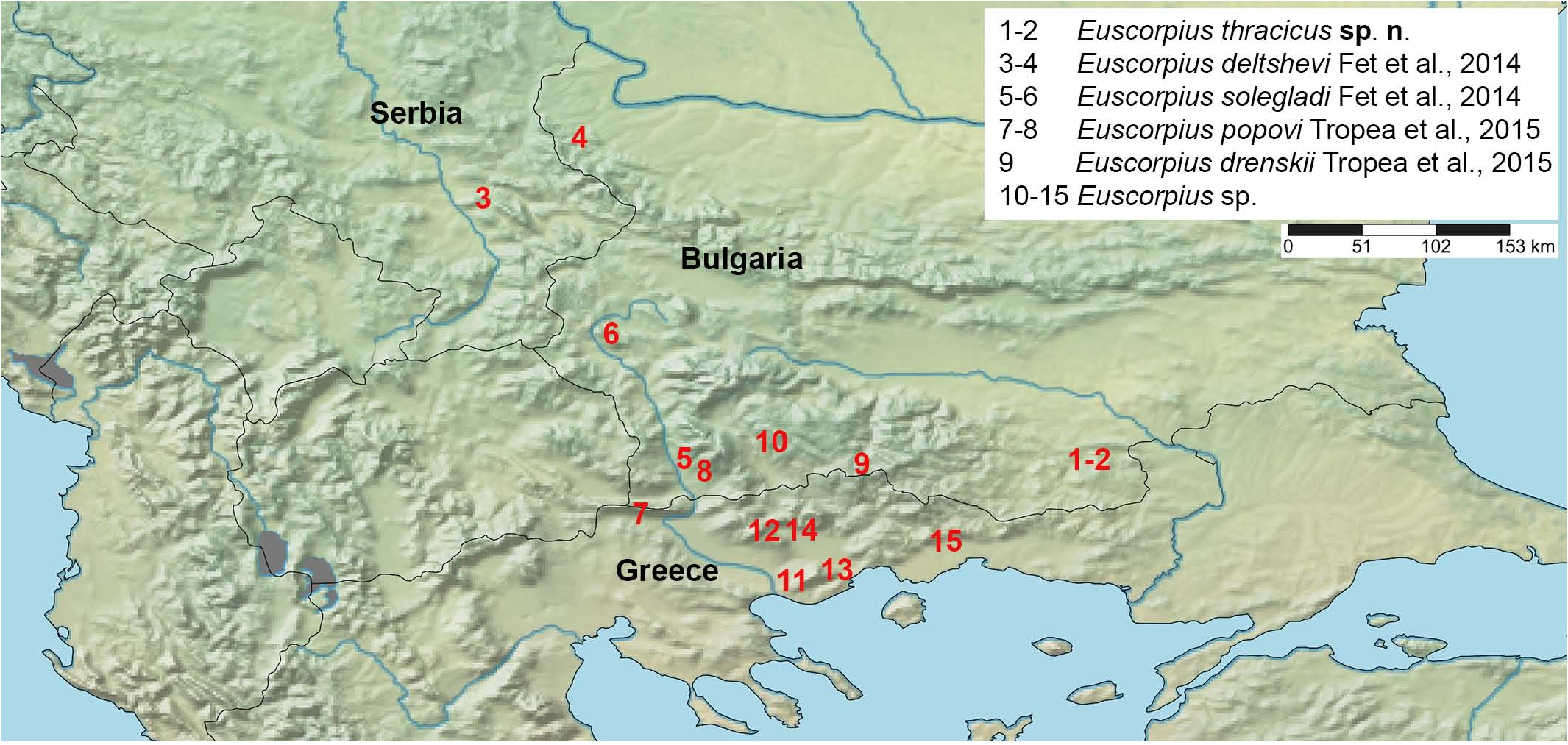
( Figures 1–49 View Figures 1–2 View Figures 3–6 View Figures 7–16 View Figures 17–22 View Figures 23–31 View Figures 32–39 View Figures 40–43 View Figures 44–49 , 58–61 View Figures50–58 View Figures 59–60 View Figure 61 , Tables 1–2 View Table 1 View Table 2 )
http://zoobank.org/urn:lsid:zoobank.org:act:1BDEC12C- 018B-4123-9A1A-397BA3266047
TYPE LOCALITY AND TYPE REPOSITORY. Bulgaria, Kardzhali Province, Krumovgrad Municipality, Bryagovets Village , 41.6485862°N 25.8132185°E GoogleMaps ; FKCP.
TYPE MATERIAL. Bulgaria, Kardzhali Province, Krumovgrad Municipality, BryagovetsVillage , 41.6485862°N 25.8132185°E ( Figs. 59–60 View Figures 59–60 , 1 View Figures 1–2 ♂ (holotype) 6♂ 8♀ (paratypes, Nos. 1864, 1865, 1866, 1878, 1879, 1882, 1883), 25 September 2020, leg. M. Byronová et al., GoogleMaps FKCP.
ETYMOLOGY. Named after Thracia (Thrace), the ancient name of the region where the type locality is situated.
DIAGNOSIS (♂ ♀). Total length 28–32 mm. Color reddish yellow to brown, telson yellow. Pedipalp patella external trichobothria numbers: 4 eb, 4 eb a, 2 esb, 4 em, 4 est, 6 et, ventral aspect of patella with 7 trichobothria. Pectinal teeth number 8–10 in males, 6–9 in females. Chelicerae yellow, very slightly reticulated. Male with pedipalp finger marginal profile type C (KovařÍk & Šťáhlavský, 2020: 2), female with fingers very weakly undulate, almost linear. Dorsal metasomal carinae on segments III– IV irregularly granulated, mainly in male. Dorsolateral carinae on metasomal segments II–IV absent. Ventrolateral carinae on metasomal segments II–IV present or indicated and smooth. Metasoma V ventrally with median carina indicated. Metasoma finely granulated dorsally in male and smooth in female. Chela length/width ratio, 2.35–2.48 in male, 2.50–2.55 in female. Metasoma IV length/width ratio, 1.8–1.9 in both sexes. Telson length/depth ratio, 2.5–2.6 in male, 3.1–3.2 in female.
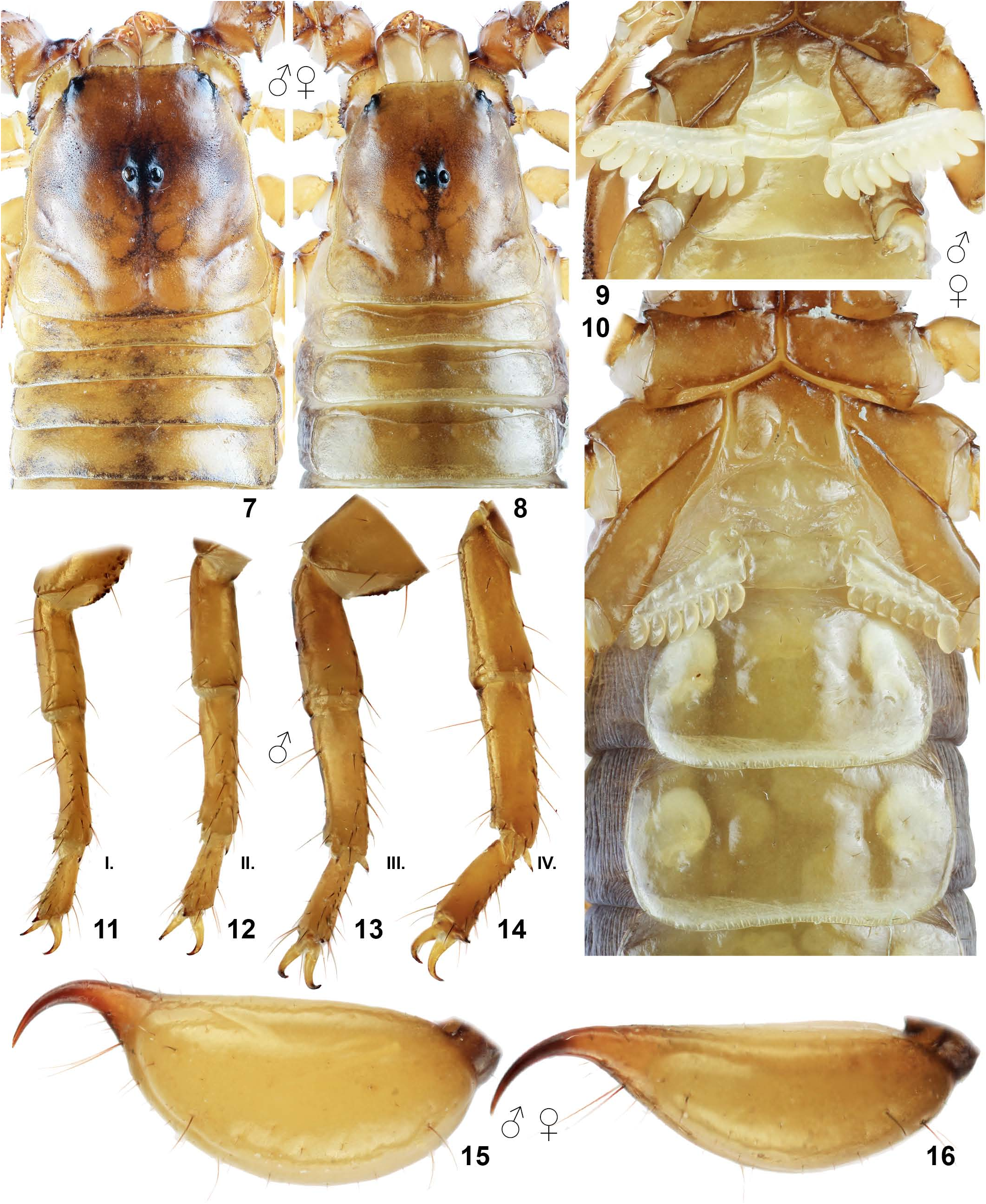
DESCRIPTION (♀ ♂). Total length 28–32 mm in both sexes. The habitus is shown in Figs. 1–6 View Figures 1–2 View Figures 3–6 . For position and distribution of trichobothria on pedipalps, see Figs. 32–38 View Figures 32–39 . For sexual dimorphism, see below in the description. For measurements, see Table 1 View Table 1 .
Coloration ( Figs. 1–6 View Figures 1–2 View Figures 3–6 ). Base color uniformly reddish yellow to brown including sternites, telson yellow, pedipalps reddish brown. Chelicerae yellow and very slightly reticulate.
Carapace and mesosoma ( Figs. 7–10 View Figures 7–16 ). Carapace finely granulated with several smooth areas; carinae absent. Anterior margin of carapace straight. Carapace with two lateral eyes. Tergites finely granulated, more so in male, without carinae developed. Tergite VII lacking median and paired lateral carinae. Sternites III–VII smooth and lustrous; VII lacking median and paired lateral carinae. Stigmata small, narrow ellipical. Pectinal teeth number 8–10 (8 x 8, 4 x 9, 2 x 10) in males and 6–9 (1 x 6, 12 x 7, 2 x 8, 1 x 9) in females, fulcra present. Pectines with 3 marginal lamellae and 4–6 middle lamellae.
Metasoma and telson ( Figs. 15–22 View Figures 7–16 View Figures 17–22 ). Metasoma very sparsely hirsute and smooth. Metasoma I–V finely granulated in male, several fine granules also present on lateral surfaces of metasoma I and V in both sexes. Dorsal carinae on metasomal segments I–V irregularly granulated in male, reduced in female; dorsolateral carinae absent; ventrolateral carinae present or indicated and smooth on segments II–IV, granulated on segment V. Metasoma V ventrally granulated with median carina indicated, metasoma I–IV with ventral median carinae absent.Anal arch with small pigmented granules. Telson rather smooth, elongate in female and swollen in male, with annular ring indicated in female and developed in male. Aculeus short, more curved in male.
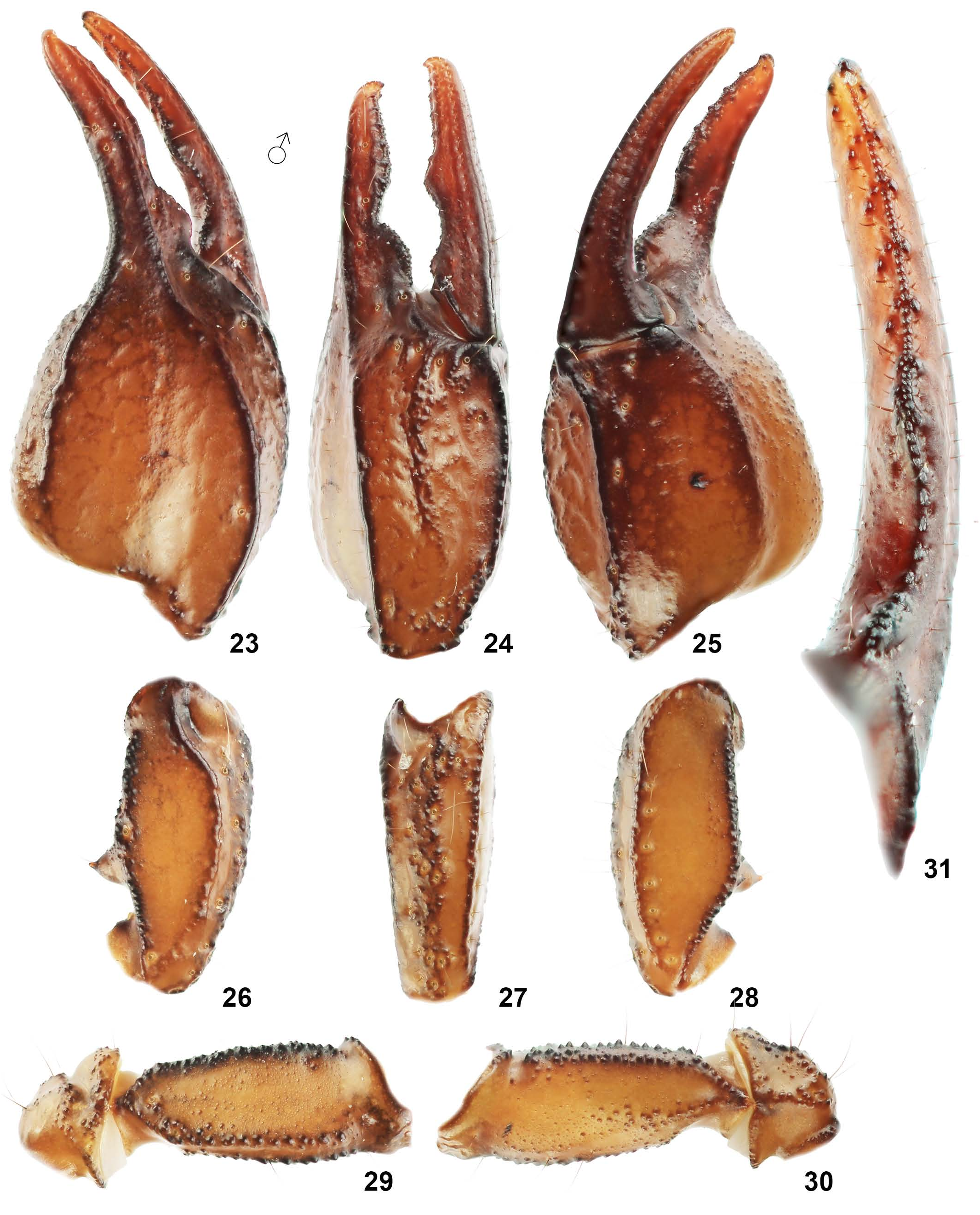
Pedipalps ( Figs. 23–39 View Figures 23–31 View Figures 32–39 ). Pedipalps very sparsely hirsute. Patella with 24 (4 eb, 4 eb a, 2 esb, 4 em, 4 est, 6 et) external and 7 ventral trichobothria. Chela with 4 trichobothria in ventral series, of which V 4 is located external to the ventroexternal carina, on the external surface near Eb 1. Entire femur finely granulated and patella smooth with fine granulated dorsointernal and ventrointernal margins. Femur with granulated developed carinae; ventroexternal carina incomplete. Patella with 5 complete carinae including irregular wide externomedian carina. Dorsal patellar spur well developed in male. Manus dorsally with fine, rounded granules, which do not form a median carina (mainly in male); only five chelal carinae developed. Male with pedipalp finger undulation profile type C, female with fingers very weakly undulate, almost linear.
Legs ( Figs. 11–14 View Figures 7–16 ). Both pedal spurs present on all legs, lacking spinelets; tibial spurs absent. Tarsus with single row of spinules on ventral surface, terminating distally with two essentially adjacent spinules.
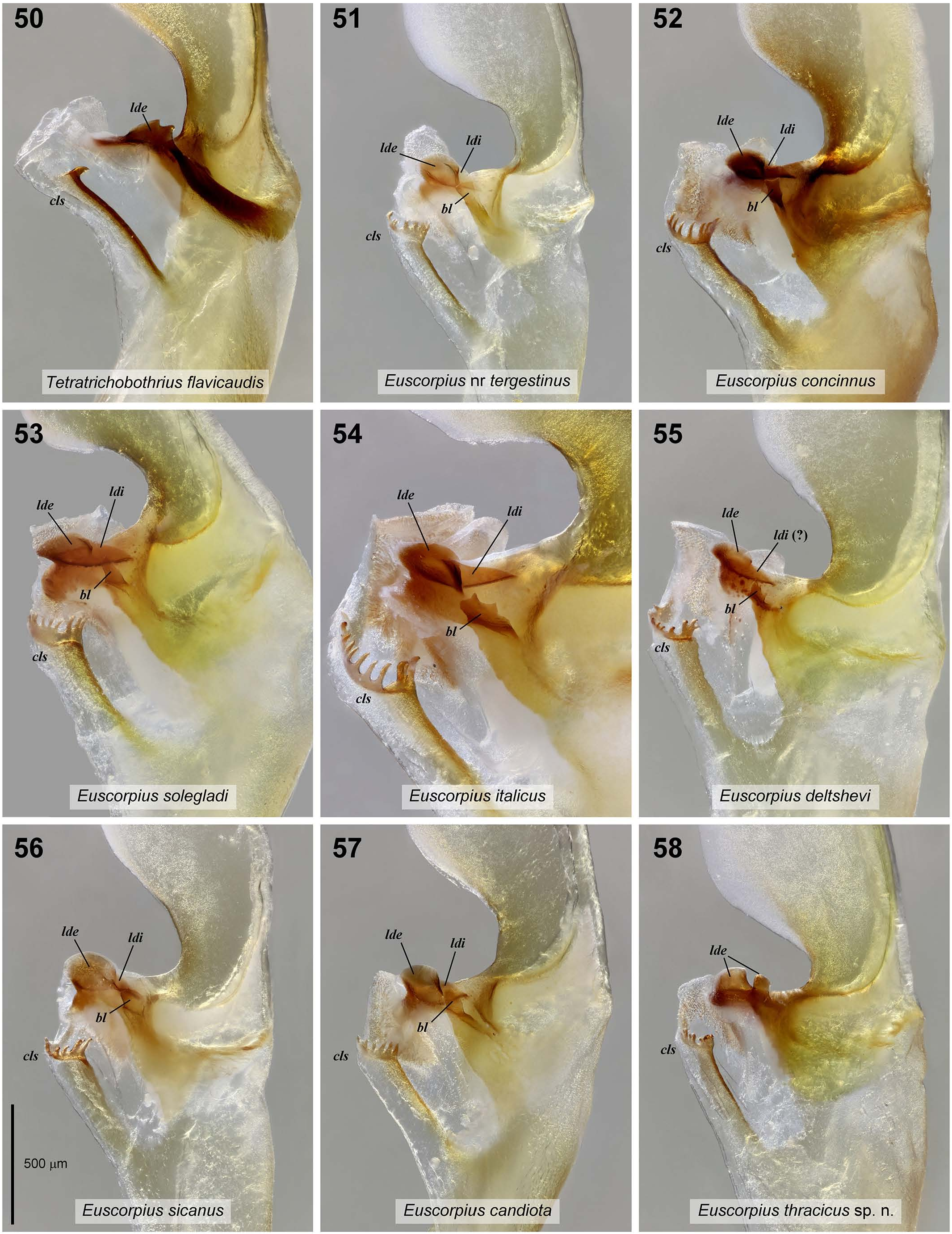
Hemispermatophore ( Figs. 44–58 View Figures 44–49 View Figures50–58 ). Lamelliform. Shape and proportions typical of the genus (Fet & Soleglad, 2002). Distal lamina proximally broad, tapered distally, with strong basal constriction to half maximal width at base. Apex of lamina narrow but blunt, bent posteriorly. Capsule broad, with transverse trough on convex surface. Distal margin of trough strengthened with transverse rib that curves and connects to posterior axial rib of distal lamina. Truncal flexure well developed, separating trunk from capsule. Trunk relatively short, ca. 2/3 of length of distal lamina, gradually tapered towards base. Pedicel almost as long as trunk. Basal carina of capsule weakly sclerotized, terminating in typical crownlike structure (cls) with 4–7 hook-like tines. Distal carina of capsule strongly sclerotized, distal external lobe (lde) apparently divided into two cristate ‘sub-lobes’, one broader and one narrower, separated by a deep cleft. Distal internal lobe (ldi) apparently absent. Basal lobe (bl) absent. Terminal membrane of sperm duct with numerous fine spicules. Both left and right hemispermatophores from 3 individuals (paratypes No. 1864, 1865 and 1866) displayed similar morphology. Measurements of left hemispermatophore No. 1864 (mm): distal lamina length from base of basal constriction to apex 2.50, maximal width 0.78; trunk length from truncal flexure to pedicel 1.34; pedicel length 1.06. Variation: cls tine counts (with bifid tines counting as 2, trifid as 3, etc.) varied among 3 examined paratypes: No. 1864, right 4, left 7; No. 1865, right 4, left 5; No. 1866, right 6, left 4.
Karyotype ( Figs. 40–43 View Figures 40–43 ). We analyzed the chromosomes of four paratype males (Nos. 1864, 1965, 1866, 1878). The diploid complement of these samples is composed of 92 chromosomes ( Figs. 40, 41 View Figures 40–43 ). In all observed postpachytene and metaphase I nuclei, we observed 46 bivalents without visible chiasmata ( Fig. 42 View Figures 40–43 ). The relative length of the chromosomes of the diploid set decreased gradually from 2.23% to 0.37%. The chromosomes exhibited monocentric organization and the morphology of all chromosome pairs was telocentric with only one exception. The chromosomes of the pair number 18 were metacentric. The arm ratio of chromosomes belonging to this pair was 1.17 (the standard deviation is 0.12). These two biarmed chromosomes were clearly visible during postpachytene ( Fig. 42 View Figures 40–43 ) as well as during metaphase II ( Fig. 43 View Figures 40–43 ).
The number of chromosomes in E. thracicus sp. n. (2n=92) fully corresponds to the known 2n values of two Euscorpius species from the Balkan region ( E. janstai from North Macedonia: 2n=112 and E. sadileki from Serbia: 2n=90) (KovařÍk & Šťáhlavský, 2020). However, the karyotypes of these species differ conspicuously from that of E. thracicus sp. n. by the presence of a higher amount of biarmed chromosomes in their karyotypes. Our cytogenetic results from the Balkan Peninsula document a high karyotype variability in the genus Euscorpius in this region. We support the application of this marker in taxonomy of the family Euscorpiidae , as already implemented in the genus Alpiscorpius (Euscorpiidae) from the Alps ( Štundlová et al., 2019).
DNA analysis ( Table 2 View Table 2 ). The comparison of the differences among the sequences of the mitochondrial 16S rRNA or/ and COI genes within the genus Euscorpius represents one of the primary important tools for species delimitation in this morphologically uniform group (e.g. Gantenbein et al., 1999; Parmakelis et al., 2013; Štundlová et al., 2019). Our comparison of the genetic distances (p-distances and Kimura 2-parameter distances) ( Table 2 View Table 2 ) among the species known from the studied region ( Bulgaria, Eastern Serbia, and Northern Greece) corresponds to the previous analyses of the species from this area ( Parmakelis et al., 2013; Fet et al., 2014; Tropea et al., 2015 a, 2015 b) and retrieved values of distances always exceeded 0.046 among all analyzed species. This fact supports a separate position and species status of E. thracicus sp. n.
AFFINITIES. DNA analysis separates E. thracicus sp. n. from all other species of the genus. The morphologically most similar species is E. popovi Tropea et al., 2015 , from southwestern Bulgaria and northeastern Greece. These two species are differentiated according to the granulation of the dorsal surface of the pedipalp patella, which is well developed in E. popovi and reduced to absent in E. thracicus sp. n. ( Fig. 26 View Figures 23–31 versus fig. 9 in Tropea et al., 2015 a: 5). The male of E. thracicus sp. n. has pedipalp finger margin profile type C versus type A in E. popovi . Females have fingers very weakly undulate, almost linear in E. thracicus sp. n. and undulate in E. popovi ( Figs. 24 View Figures 23–31 , 33 View Figures 32–39 vs. Figs. 7, 11 View Figures 7–16 in Tropea et al., 2015 a: 5; see KovařÍk & Šťáhlavský, 2020: 2).
The hemispermatophore distal carina of E. thracicus sp. n. differed from those of other examined species of related euscorpiids (cf. Fig. 58 View Figures50–58 vs. Figs. 50–57 View Figures50–58 ) in: (i) lacking a basal lobe (bl), (ii) having the distal external lobe (lde) apparently divided into two separate sub-lobes, and (iii) lacking the distal internal lobe (ldi). An alternative interpretation of the anatomy is that the broader distal sub-lobe corresponds to the lde, and the narrower distal sub-lobe to the ldi. However, in other species the ldi is always directly joined to the lde by an oblique non-terminal suture (which may be partial or limited to a short notch), and its axis is distinctly slanted relative to the lde axis. In contrast, the two sub-lobes observed here were strongly separated from each other with their axes nearly parallel. We also reject the hypothesis that the narrower distal sub-lobe is homologous to the basal lobe (bl), since the bl invariably arises from a more basal position on the distal carina in other species.
Of 33 other species of related euscorpiids with described hemispermatophores, the bl was absent in only one species, Tetratrichobothrius flavicaudis (De Geer, 1778) ( Fig. 50 View Figures50–58 ; Fet & Soleglad, 2002; Jacob et al., 2004b; Vachon, 1948). It has been reported to be present in 6 species of Alpiscorpius Gantenbein et al., 1999 ( A. alpha (Di Caporiacco, 1950) ; A. gamma (Di Caporiacco, 1950) ; A. germanus (C.L. Koch, 1837); A. mingrelicus (Kessler, 1874) ; A. phrygius (Bonacina, 1980); A. uludagensis (Lacroix, 1995)) ( Jacob et al. 2004b; Molteni et al., 1983; Scherabon, 1987; Tropea et al., 2015 c), and in 26 species of Euscorpius ( E. avcii Tropea et al., 2012 ; E. balearicus Di Caporiacco, 1950 ; E. birulai Fet et al., 2014 ; E. candiota Birula, 1903 ; E. carpathicus (Linnaeus, 1767) ; E. ciliciensis Birula, 1898; E. concinnus (C. L. Koch, 1837) ; E. deltshevi Fet et al., 2014 ; E. drenskii Tropea et al., 2015 ; E. feti Tropea, 2013; E. giachinoi Tropea & Fet, 2015 ; E. gocmeni Tropea et al., 2014; E. hadzii Di Caporiacco, 1950 ; E. italicus (Herbst, 1800) ; E. kinzelbachi Tropea et al., 2014 ; E. kritscheri Fet et al., 2013; E. lycius Yağmur et al., 2013 ; E. mylonasi Fet et al., 2014; E. oglasae Di Caporiacco, 1950 ; E. sicanus (C. L. Koch, 1837) ; E. solegladi Fet et al., 2014 ; E. stahlavskyi Tropea et al., 2014; E. tauricus (C. L. Koch, 1837) ; E. tergestinus (C.L. Koch, 1837) ; E. sp. cf. tergestinus ; E. vailatii Tropea & Fet, 2015 ) ( Figs. 51–57 View Figures50–58 ; Fet & Soleglad, 2002; Fet et al., 2013; Fet et al., 2014; Gantenbein et al., 2002; Jacob et al, 2004a, 2004b; Scherabon, 1987; Tropea et al., 2014a, 2014b, 2015b, 2015c; Tropea & Fet, 2015; Tropea & Ozimec, 2019; Vignoli et al., 2007; Yağmur et al., 2013; Yağmur & Tropea, 2013). The bl was not directly reported in two other species. Gantenbein et al. (2002) described and illustrated the hemispermatophore of E. naupliensis (C. L. Koch, 1837) , which seemed to lack the bl, but their text and figure were inconclusive. Tropea et al. (2013) omitted mention of the bl in E. erymanthius , but did not exclude it, and its presence is unclear.
Absence of the ldi is another character shared between E. thracicus sp. n. and T. flavicaudis . When this lobe is weakly developed, it can be intraspecifically variable, being absent among some individuals of a species (e.g., A. alpha , A. gamma and A. germanus ; Jacob et al, 2004b). It has been reported to be present in 15 species of Euscorpius (i.e., E. balearicus Di Caporiacco, 1950 ; E. birulai Fet et al., 2014 ; E. candiota Birula, 1903 ; E. carpathicus (Linnaeus, 1767) ; E. concinnus (C. L. Koch, 1837) ; E. deltshevi Fet et al., 2014 ; E. hadzii Di Caporiacco, 1950 ; E. italicus (Herbst, 1800) ; E. kritscheri Fet et al., 2013 ; E. mylonasi Fet et al., 2014 ; E. oglasae Di Caporiacco, 1950 ; E. sicanus (C. L. Koch, 1837) ; E. solegladi Fet et al., 2014 ; E. tergestinus (C.L. Koch, 1837) ; E. sp. cf. tergestinus ) ( Figs. 51–57 View Figures50–58 ; Fet & Soleglad, 2002; Fet et al., 2013; Fet et al., 2014; Gantenbein et al., 2002; Jacob et al, 2004a, 2004b; Scherabon, 1987; Vignoli et al, 2007).
The lde appears to be almost universally present in hemispermatophores of these genera that have been described to date. However, none have been reported to exhibit apparent division into two sub-lobes. Perhaps the most proximate condition is in T. flavicaudis , whose lde is bilobate or bidentate, but undivided ( Fig. 50 View Figures50–58 ). In molecular studies, T. flavicaudis resolved as a basal lineage and outgroup that was not closely related to E. popovi ( Tropea et al., 2015 a: 11, fig. 20), which is the species most similar morphologically to E. thracicus sp. n. Thus, in E. thracicus sp. n., ldi and bl may have been independently lost much later in the evolutionary history of the group. Their absence is probably the primitive state in T. flavicaudis .
The unusual morphology of the distal carina of E. thracicus sp. n., was consistent across 6 hemispermatophores from 3 males, indicating a taxonomically stable character. This underlines the importance of accurately characterizing and illustrating these structures for comparative analysis. Hemispermatophore anatomy has yet to be reported in the majority of other related euscorpiids (9 Alpiscorpius spp., 36 Euscorpius spp.).
DISTRIBUTION. Bulgaria (eastern Rodopi Mts.) ( Fig. 61 View Figure 61 ).
COMPARATIVE HEMISPERMATOPHORE MATERIAL (♂, Figs. 50–57 View Figures50–58 ). Euscorpius concinnus (C. L. Koch, 1837) , France, Var Department , Le Thoronet, 43.459761°N 6.281816°E, No. 1642, leg. M. Stockmann. GoogleMaps
Euscorpius candiota Birula, 1903 , Greece, Crete, Lassithi Plateau GoogleMaps , 35.149605°N 25.504660°E, No. 1728, leg. M. Stockmann.
Euscorpius deltshevi Fet et al., 2014 , Bulgaria, Sofia Province, Dragoman Municipality GoogleMaps , Petrovski Krast Mtn., 42.9403326°N 22.9667976°E, 2 October 2020, No. 1867, leg. O. Vaněk.
Euscorpius italicus (Herbst, 1800) , Croatia, Istria, 2 km SE Presika , 45.066421°N 14.138763°E, No. 1646 GoogleMaps .
Euscorpius sp., cf. tergestinus , Croatia, Krk Island, No. 1481. Euscorpius sicanus (C. L. Koch, 1837) , Italy, Sicily, Syracuse Province, Noto Antica, August 2014, No. 881, leg. C. Turiel.
Euscorpius solegladi Fet et al., 2014 , Bulgaria, Blagoevgrad Province, Petrich Municipality, Rupite, Kozhuch Mt. GoogleMaps , 41.646179°N 23.361132°E, October 2020, No. 1861, leg. T. Ryšan.
Tetratrichobothrius flavicaudis (De Geer, 1778) , France, Var Department, Gonfaron, 43.341578°N 6.294433°E, No. 1350, leg. M. Stockmann.
Table 2. A matrix of genetic distances among the COI genes of the Euscorpius species from Bulgaria, eastern Serbia, and northern Greece. p-distance (below the diagonal); Kimura 2-parameter distance (above the diagonal). In the brackets are mentioned specimen codes/accession number from GenBank.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E. thracicus sp. n. (S1865/ MW291129 View Materials ) | - | 0.002 | 0.094 | 0.102 | 0.099 | 0.099 | 0.064 | 0.066 | 0.078 | 0.060 | 0.050 | 0.049 | 0.052 | 0.048 | 0.067 |
| 2 | E. thracicus sp. n. (S1866/ MW291114 View Materials ) | 0.002 | - | 0.096 | 0.104 | 0.101 | 0.101 | 0.067 | 0.068 | 0.080 | 0.062 | 0.052 | 0.052 | 0.054 | 0.050 | 0.069 |
| 3 | E. deltshevi (VF-0746-1/ KM111244 View Materials ) | 0.087 | 0.089 | - | 0.047 | 0.079 | 0.081 | 0.086 | 0.087 | 0.101 | 0.089 | 0.075 | 0.079 | 0.081 | 0.076 | 0.084 |
| 4 | E. deltshevi (VF-0821-1/ KM111243 View Materials ) | 0.094 | 0.096 | 0.045 | - | 0.089 | 0.089 | 0.097 | 0.093 | 0.106 | 0.083 | 0.092 | 0.097 | 0.099 | 0.093 | 0.086 |
| 5 | E. solegladi (VF-0801-1/ KM111247 View Materials ) | 0.093 | 0.094 | 0.074 | 0.083 | - | 0.003 | 0.088 | 0.089 | 0.112 | 0.085 | 0.081 | 0.083 | 0.085 | 0.082 | 0.092 |
| 6 | E. solegladi (VF-0802-1/ KM111246 View Materials ) | 0.093 | 0.094 | 0.076 | 0.083 | 0.004 | - | 0.090 | 0.091 | 0.115 | 0.087 | 0.083 | 0.085 | 0.087 | 0.084 | 0.090 |
| 7 | E. popovi (FESP13/ KC215733 View Materials ) | 0.061 | 0.063 | 0.081 | 0.090 | 0.083 | 0.084 | - | 0.012 | 0.091 | 0.064 | 0.059 | 0.056 | 0.058 | 0.055 | 0.082 |
| 8 | E. popovi (FESP21/ KC215737 View Materials ) | 0.063 | 0.065 | 0.082 | 0.087 | 0.083 | 0.085 | 0.012 | - | 0.086 | 0.064 | 0.056 | 0.056 | 0.058 | 0.054 | 0.078 |
| 9 | E. drenskii (117F/ KT602916 View Materials ) | 0.073 | 0.075 | 0.094 | 0.098 | 0.104 | 0.106 | 0.084 | 0.081 | - | 0.067 | 0.062 | 0.063 | 0.063 | 0.064 | 0.072 |
| 10 | E. sp. (113F/ KC215662 View Materials ) | 0.057 | 0.059 | 0.084 | 0.078 | 0.080 | 0.082 | 0.061 | 0.061 | 0.063 | - | 0.040 | 0.047 | 0.050 | 0.046 | 0.058 |
| 11 | E. sp. (FESP12/ KC215732 View Materials ) | 0.048 | 0.050 | 0.071 | 0.086 | 0.076 | 0.078 | 0.057 | 0.054 | 0.058 | 0.039 | - | 0.010 | 0.010 | 0.008 | 0.041 |
| 12 | E. sp. (FESP11/ KC215731 View Materials ) | 0.048 | 0.050 | 0.074 | 0.090 | 0.078 | 0.080 | 0.054 | 0.053 | 0.060 | 0.046 | 0.010 | - | 0.000 | 0.000 | 0.048 |
| 13 | E. sp. (FESP09/ KC215729 View Materials ) | 0.050 | 0.052 | 0.076 | 0.092 | 0.080 | 0.082 | 0.056 | 0.055 | 0.060 | 0.048 | 0.010 | 0.000 | - | 0.000 | 0.048 |
| 14 | E. sp. (FESP10/ KC215730 View Materials ) | 0.046 | 0.048 | 0.071 | 0.087 | 0.077 | 0.079 | 0.052 | 0.052 | 0.060 | 0.044 | 0.008 | 0.000 | 0.000 | - | 0.047 |
| 15 | E. sp. (120F/ KC215663 View Materials ) | 0.063 | 0.065 | 0.079 | 0.081 | 0.086 | 0.084 | 0.077 | 0.073 | 0.067 | 0.056 | 0.039 | 0.046 | 0.046 | 0.045 | - |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |