Akodon polopi, J. Pablo Jayat, Pablo E. Ortiz, Jorge Salazar-Bravo, Ulyses F. J. Pardiñas & Guillermo D’Elía, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.293461 |
|
DOI |
https://doi.org/10.5281/zenodo.6196161 |
|
persistent identifier |
https://treatment.plazi.org/id/E91F87ED-FFA2-FF8F-D9DA-890DFDE9FAA2 |
|
treatment provided by |
Plazi |
|
scientific name |
Akodon polopi |
| status |
sp. nov. |
Akodon polopi , new species
Akodon sp. sensu Polop, 1989:53. Studies on Neotropical Fauna and Environments 24:53–59.
Akodon boliviensis sensu Polop, 1991:115 . Revista de la Universidad Nacional de Río Cuarto 11:115–121.
Akodon sp. sensu Pinna-Senn et al., 1992 Mendeliana 10: 59–70.
Akodon alterus sensu Priotto et al., 1996:135 . Facena 12: 135–138.
Akodon boliviensis sensu Morando & Polop, 1997:132 . Mastozoología Neotropical 4 (2): 129–136.
Akodon spegazzinii sensu D’Elía, 2003: 310 . Cladistics 19:307–323.
Akodon spegazzinii sensu D’Elía et al., 2003:354 . Mammalian Biology 68:351–364.
Akodon spegazzinii sensu Kufner et al., 2004:120 . Ecología Aplicada 3 (1,2):118–121.
Akodon spegazzinii sensu Pardiñas et al., 2005: 473 . Journal of Mammalogy 86 (3):462–474.
Akodon spegazzinii sensu Rodrigues Gonçalvez et al., 2007: 23 . Miscellaneous Publications of the Museum of Zoology, University of Michigan 197: 1–24.
Akodon spegazzinii sensu Smith & Patton, 2007: 831 . University of California Publications in Zoology 134:1–981.
Akodon sp. sensu Jayat et al., 2007a: 203. Mastozoología Neotropical 14 (2):201–225.
Holotype: MACN 23486, Adult male (age class 4), collected by J. Pablo Jayat, Pablo E. Ortiz, Daniel García Lopez, and Rodrigo Gonzalez on August 17, 2008 (original field number JPJ 2125), skin, skull, skeleton and tissues in alcohol ( Figs. 12 View FIGURE 12 and 13 View FIGURE 13 ).
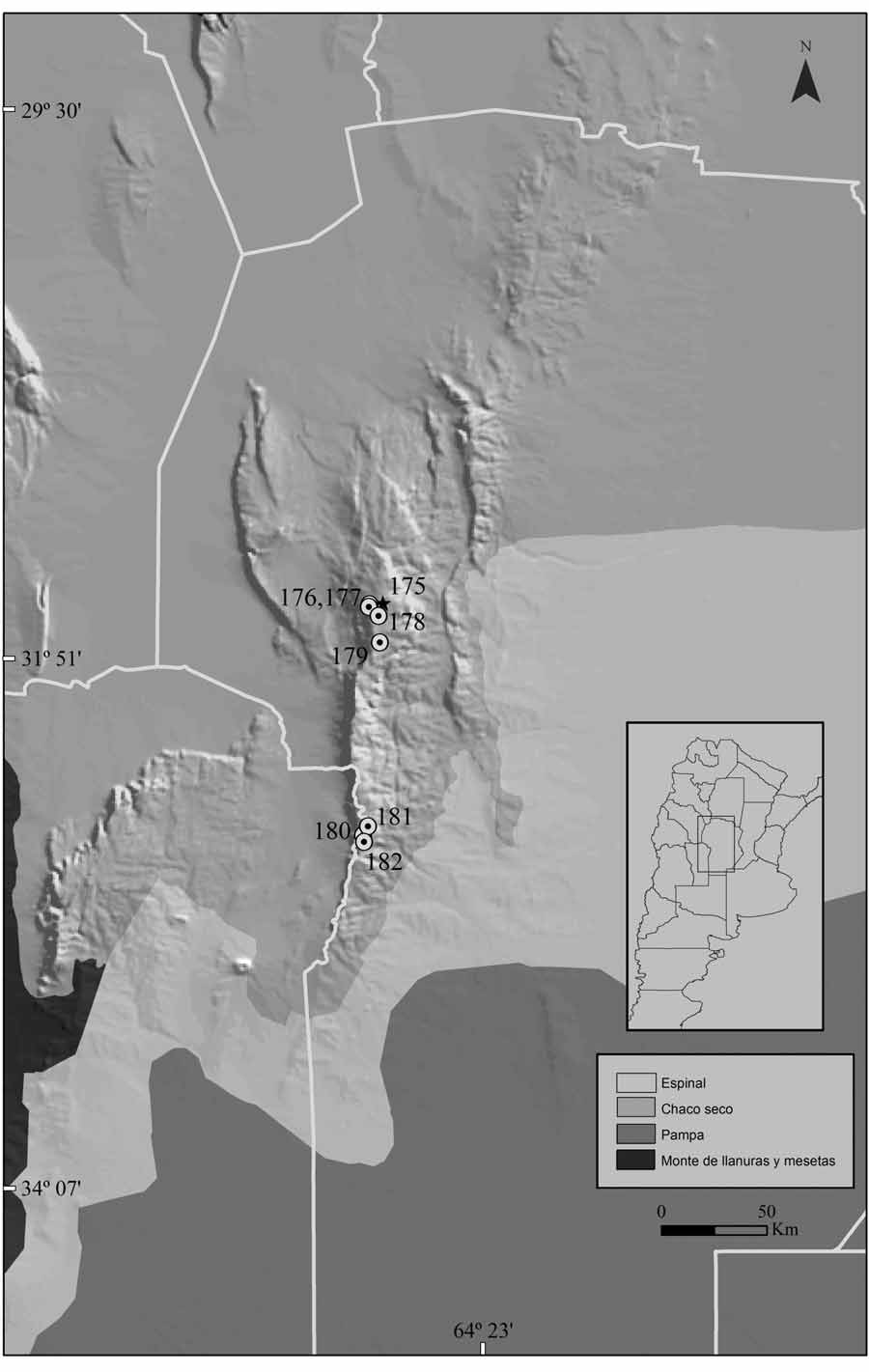
Type locality: Pampa de Achala, 6 km E (by highway 34) from antena repetidora La Posta, 2200 m (31º 36’ 44.5” S, 64º 48’ 48.7” W), San Alberto Department, Córdoba Province, Argentina ( Fig. 14 View FIGURE 14 ).
Diagnosis: A member of the Subfamily Sigmodontinae distinguishable from all other species of Akodon by the following combination of characters: size intermediate for the genus (mean values in mm for individuals of age class 4; length of head and body, 100; tail length, 70; condyloincisive length, 24.53; maxillary toothrow length, 4.40); fur dense and soft; general coloration uniform, buffy brown; chin with a small but distinguishable white patch; claws on fore and hind feet somewhat long (mean values in mm for 10 individuals of age class 4 in the medial finger: 2.81 and 3.26 respectively); skull with the rostrum relatively short and broad; interorbital region hour-glass shaped but with sharply squared posterior margins; temporal and lambdoid ridges well developed; zygomatic plate relatively broad; mesopterygoid fossa narrow. Upper incisors slightly proodont; first lower molar with a conspicuous metastylid and mesostylid. Molecular apomorphies are listed in Table 13 View TABLE 13 (note that sequences of some Akodon species were not analyzed, and that only three haplotype of Akodon polopi , new species were available; therefore, these character states should be taken with caution).
Measurements of the holotype: External measurements (in mm): length of head and body, 103; tail length, 76; length of hind foot (with claw), 25; ear length, 15; weight (in g): 35. Cranial measurements (in mm): greatest length of skull, 26.80; condyloincisive length, 25.50; zygomatic breadth, 13.84; braincase breadth, 11.84; interorbital constriction, 4.58; maxillary toothrow length, 4.60; nasal length, 10.10; mid rostral width, 5.00; diastema length, 7.12; length of incisive foramen, 6.54; width across occipital condyles, 6.70; breadth of zygomatic plate, 2.50. See Table 14 View TABLE 14 for measurements of paratypes.
Paratypes: Seven specimens collected at the type locality (CNP 1927, 1928; CML 7672, 7673; and MACN 23487, 23488, 23489) ( Table 14 View TABLE 14 ).
Other referred specimens: Twenty one specimens from the type locality (JPJ 2118, 2120, 2121, 2123, 2126 to 2129, 2131, 2133 to 2135, 2139, 2141, 2144, 2146, 2147, 2149, 2150, 2158, 2159); four specimens from Pampa de Achala ( CUNRC 2805, 10145, 10178, 50151); three specimens from Pampa de Achala, 2163 m ( CUNRC 44748, 44749, 44750); three specimens from Pampa de Achala, 2247 m ( CUNRC 44744, 44745, 44747), and nine specimens from Repetidora La Posta, Pampa de Achala, 2171 m (CNP 1500 to 1508).
Distribution: The new species is only known from few localities in Córdoba Province, Argentina: Pampa de Achala, a highland plateau situated in the Sierras Grandes ( Polop 1989, 1991), three localities in Río Cuarto Department (Cerro de Oro, Puesto Gonzalez and La Ventana, all above 1500 m elevation; Priotto et al. 1996), and two additional sites in Pampa de San Luis, Cruz del Eje Department (SW of Pampa de San Luis, 1900 m, and near Cuchilla Nevada, 1700-1800 m; Kufner et al. 2004) ( Fig. 14 View FIGURE 14 ).
Etymology: Dedicated to our friend and colleague Jaime José Polop (Universidad Nacional de Río Cuarto, Córdoba, Argentina) for his invaluable contributions to the understanding of the ecology of sigmodontine rodents from central Argentina. In addition, Jaime collected many of the specimens used in the characterization of the new species and even pointed-out the distinctiveness of this form ( Polop 1989: 58).
Morphological description: Fur dense and soft. Dorsal coloration uniform, buffy brown lightly spattered with black hairs. Guard hairs generally black excepting those from the rump, which are distally whitish. In this region, the guard hairs extend beyond the level of the fur hairs by approximately 5 mm. Flanks coloration clearer and more richly colored. Ventral side clearly contrasting with dorsum and flanks, with buffy or tawny tinges. Chin with a small but distinguishable white patch. Ears densely covered by hairs of the same general color as the dorsum. Fore and hind feet whitish or buffy and densely furred. Tail clearly bicolored, blacky brown dorsally and whitish ventrally. Claws on fore and hind feet somewhat longer than in the other species of the boliviensis group and densely covered by a whitish tuft.
Skull heavily constructed in the context of the boliviensis group, with the rostrum relatively short and broad. Nasals short, not acuminate, and extended almost to the anterior face of incisors; frontal sinuses clearly inflated and zygomatic notches broad and deep. Interorbital region hour-glass shaped, with rounded or lightly squared borders and with posterior margins having a greater tendency to be sharply squared than is usually the case for the boliviensis group. Temporal and lambdoid ridges well developed for this group. Zygomatic plate relatively broad, with its anterior margin straight and vertical in most of the individuals. Hamular process relatively robust but showing a variable development, with distal end expanded. Incisive foramina long, with posterior ends reaching the hypoflexus of M1. Mesopterygoid fossa narrow, with its anterior margin rounded and lateral borders slightly divergent backward. Posterior palatal pits small and variable in position. Parapterygoid fossae slightly excavated and broader than mesopterygoid fossa, with lateral margins generally straight and divergent posteriorly. Auditory bullae of intermediate size for the genus, with short and wide Eustachian tubes. Mandible similar to the remaining species of the boliviensis group but somewhat more robust, with the horizontal ramus higher and the coronoid process broader. The capsular projection clearly posterior to the coronoid process. Anterior point of diastema located below the alveolar plane. The angular process ends just ahead the condyloid process. Masseteric crest reachs the level of the anterior margin of m1 or slightly behind.
Teeth of typical Akodon pattern ( Fig. 15 View FIGURE 15 ). Upper incisors approximately orthodont, but many individuals somewhat proodont. M1 with procingulum and anteromedian flexus well developed. A small anteroloph and mesoloph are present on the labial side and on the lingual side a tiny enteroloph is visible in some young specimens. M2 with a reduced procingulum and a vestigial mesoloph present. The posteroflexus not well developed. M3 shows the paraflexus and metaflexus always present in young specimens. The hypoflexus is present in only a few individuals. Lower molars crested and transversally compressed. The m1 has a well developed procingulum with a deep anteromedian flexid and a clearly defined anterolabial cingulum. All the young individuals bear a well-developed ectostylid. On the lingual side, young individuals (age classes 1 and 2) show a well-developed metastylid and a relatively robust mesolophid. The m2 preserves an anterolabial cingulum and a small ectostylid but the mesolophid is vestigial. The m3 is large and “S” shaped.
Akodon polopi has 13-14 thoracic ribs; the vertebral column includes 13-14 thoracic, 8 lumbar, and 26-27 caudal vertebrae (n = 6).
Karyotype: 2n = 40. The autosomal pairs 1 to 18 are telocentric and the pair 19 is metacentric. The X chromosomes are subacrocentric and the Y is small metacentric ( Polop 1989; Pinna-Senn et al. 1992).
Variation: In spite of marked uniformity in fur coloration, we observed slightly darker and more richly colored specimens. Some individuals have a more slender zygomatic plate, with a slightly concave anterior margin, which determine shallower zygomatic notches. The hamular process also shows a variable development, more delicate in some specimens. The posterior palatal pits also vary in position with regard to the anterior border of the mesopterygoid fossa.
Comparisons: Akodon polopi is one of the largest and more robust species of the A. boliviensis group. The species is distinguishable from the remaining species by their denser and softer fur, a large skull, with broad rostrum, deep and broad zygomatic notches, and well-expanded zygomatic arches. However, the new species has a comparatively short rostrum, narrow interorbital constriction and a not inflated braincase. A comparatively well developed metastylid in m1 of young individuals is another distinctive feature of A. polopi .
In addition to the previously mentioned characteristics, the new species can be differentiated from Akodon boliviensis by the interorbital region with its posterior margins sharply squared, temporal and lambdoid ridges well developed, and the M3 that does not show an “8” shape. The numerous morphometric differences between these species include 17 of the 20 measurements analyzed ( Table 2 View TABLE 2 ). Cyt b haplotypes of A. polopi and A. boliviensis differ by an average of 5.0% ( Table 12 View TABLE 12 ). Akodon boliviensis was registered in Argentina only in its northernmost end, always above 2400 m, whereas A. polopi lives below 2300 m on isolated mountain ranges from central Argentina, almost 900 km toward the south.
Like A. boliviensis , Akodon caenosus does not have a sharply squared interorbital posterior region or well developed temporal and lambdoid ridges. A. caenosus is unmistakable because it is situated at the opposite end in the morphometric range values, with no overlap in most of the analyzed measurements ( Table 1 View TABLE 1 ) being all of them statistically different ( Table 2 View TABLE 2 ). Likewise, cyt b haplotypes of A. polopi and A. caenosus are very divergent (6.2%).
A. polopi can be differentiated from Akodon spegazzinii by many characteristics. The former has an interorbital region with posterior margins more sharply squared, temporal and lambdoid ridges more developed and a proportionally shorter molar series. The morphometric differences between these species include 14 of the 20 analyzed measurements ( Table 2 View TABLE 2 ). Cyt b haplotypes of A. spegazzinii and A. polopi differ by on average 5.5%.
Akodon sylvanus is very similar to A. polopi in many of the variables we measured, with less than 50% (9 of 20) significantly different. However, the DA efficiently separated both species and no reciprocally misclassified specimens occurred. The anterior region of the skull of A. polopi is shorter and more robust, the zygomatic notches are deeper and broader, and the braincase is less inflated. In ventral view, the mesopterygoid fossa in A. polopi is narrower, and the upper incisors tend to be more prodont. The cyt b haplotypes of A. sylvanus and A. polopi differ on average by 4.7 %. Like A. boliviensis , A. sylvanus appears to show a disjunct distribution with regards to A. polopi ; thus far, A. sylvanus is known from only the neighborhood of its type locality in the Sierra de Santa Bárbara in Yungas forest habitats almost 800 km to the north of the type locality of A. polopi .
Natural history: The habitat in Pampa de Achala is characterized by extensive highland grasslands dominated by Festuca and Stipa , between 1800 and 2300 m elevation ( Fig 16 View FIGURE 16 ). Intermingled with the grasslands there are scattered small patches of woodlands of Polylepis australis , Heterothalamus alienus , Eupatorium buniifolium , Berberis ruscifolia, Baccharis myrtilloides and Cassia hockeriana , and rocky outcrops ( Polop 1989, 1991). None of the specimens that we captured during the end of winter 2008 (August) showed signs of reproductive activity. These data suggest that the species is reproductively active in the late spring and summer seasons and agree with previous studies where the largest number of pregnancy were in Novembre and December ( Polop 1989). This author registered an average number of embryos per female of 4.7 (range 3 to 7). Few of the specimens captured in winter show signs of pelage molting. Other sigmodontine species registered at the type locality and surroundings, in the same habitat, include Oxymycterus rufus (as O. paramensis in Polop 1989 ), Oligoryzomys flavescens , Phyllotis xanthopygus , and Reithrodon auritus . Akodon polopi , new species is the dominant cricetid in the places where it was registered. In previous studies ( Kufner et al. 2004) and our surveys, it constituted more than 70% of the captured animals but Polop (1989, 1991) indicated a minor percentage (53% and 34% respectively).
Comments: Although early considered as an undescribed species ( Polop 1989), Akodon polopi was alternatively treated as A. boliviensis ( Polop 1991; Morando & Polop 1997), A. alterus ( Priotto et al. 1996) or A. spegazzinii (D’Elía 2003; D’Elía et al. 2003; Kufner et al. 2004; Pardiñas et al. 2005; Rodrigues Gonçalvez et al. 2007 and Smith & Patton 2007).
TABLE 13. Molecular synapomorphies of Akodon polopi new species as revealed by maximum parsimony analysis of cyt b gene sequences (801 base pairs). Molecular transformations were optimized on a strict consensus tree of the 220 most parsimonious trees (1563 steps; CI = 0.287) resulted from the analysis of a cyt b matrix of 71 sequences of Akodon. Seventeen fixed derived character states were found in A. polopi new species. Of these, one derived character state, which is indicated by an asterisk, has not evolved independently in any other species of Akodon; this character has a consistency index <1 because other character states (not the one present in Akodon polopi new species) has evolved independently in more than one clade of Akodon. The remaining 16 derived character states of A. polopi new species have also secondarily appeared in at least 1 species of Akodon.
| Nucleotide position / Codon position | Character State in Akodon polopi new species | Character consistency index | |
|---|---|---|---|
| 1 | 18/3 | G | 0.333 |
| 2 | 48/3 | T | 0.200 |
| 3 | 54/3 | T | 0.091 |
| 4 | 63/3 | T | 0.429 |
| 5 | 75/3* | G | 0.286 |
| 6 | 222/3 | T | 0.167 |
| 7 | 384/3 | C | 0.167 |
| 8 | 387/3 | G | 0.400 |
| 9 | 444/3 | C | 0.056 |
| 10 | 513/3 | T | 0.125 |
| 11 | 516/3 | G | 0.200 |
| 12 | 579/3 | T | 0.200 |
| 13 | 580/1 | T | 0.143 |
| 14 | 615/3 | T | 0.167 |
| 15 | 618/3 | T | 0.143 |
| 16 | 717/3 | T | 0.333 |
| 17 | 786/3 | T | 0.500 |
TABLE 14. Measurements of seven paratypes of Akodon polopi, new species (age class 4). Abbreviations as provided in text.
| Paratypes | |||||||
|---|---|---|---|---|---|---|---|
| Variable | CNP 1927 | CNP 1928 | CML 7672 | CML 7673 | MACN 23487 | MACN 23488 | MACN 23489 |
| TBL | 172 | 150 | 173 | (181) | 180 | 179 | 164 |
| TL | 76 | 60 | 72 | (72) | 74 | 71 | 68 |
| HF | 25 | 22 | 23 | 25 | 24 | 23 | 23 |
| EL | 16 | 15 | 14 | 15 | 15 | 15 | 14 |
| W | 27.5 | 16.0 | 25.0 | 27.0 | 36.5 | 26.0 | 20.5 |
| MSL | 26.30 | 24.00 | 25.86 | 27.02 | 26.90 | 26.00 | 25.14 |
| CIL | 25.16 | 22.78 | 24.64 | 25.44 | 25.36 | 24.70 | 23.64 |
| RL | 9.62 | 8.74 | 9.34 | 9.94 | 10.18 | 9.66 | 9.30 |
| ZB | 13.50 | 12.64 | 13.38 | 13.24 | 13.40 | 13.52 | 12.86 |
| BB | 11.46 | 11.54 | 11.78 | 11.48 | 11.58 | 11.46 | 11.14 |
| IOC | 4.64 | 4.42 | 4.56 | 4.38 | 4.66 | 4.66 | 4.30 |
| MTRL | 4.30 | 4.14 | 4.62 | 4.26 | 4.44 | 4.40 | 4.54 |
| NL | 9.28 | 8.30 | 8.98 | 9.94 | 9.92 | 9.14 | 9.28 |
| RW2 | 5.00 | 4.50 | 4.88 | 5.00 | 5.20 | 5.00 | 4.68 |
| DL | 7.30 | 6.52 | 6.98 | 7.18 | 7.18 | 7.06 | 6.50 |
| IFL | 6.44 | 5.70 | 6.20 | 6.22 | 6.48 | 6.50 | 6.00 |
| OCW | 6.72 | 6.48 | 6.56 | 6.74 | 6.54 | 6.40 | 6.04 |
| ZP | 2.46 | 2.24 | 2.46 | 2.60 | 2.60 | 2.46 | 2.32 |
| ML | 14.36 | 13.22 | 13.90 | 14.28 | 14.00 | 13.94 | 13.40 |
| MdTRL | 4.28 | 4.30 | 4.56 | 4.44 | 4.44 | 4.42 | 4.52 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Akodon polopi
| J. Pablo Jayat, Pablo E. Ortiz, Jorge Salazar-Bravo, Ulyses F. J. Pardiñas & Guillermo D’Elía 2010 |
Akodon spegazzinii sensu Rodrigues Gonçalvez et al., 2007: 23
| Rodrigues Goncalvez et al. 2007: 23 |
Akodon spegazzinii sensu
| Smith & Patton 2007: 831 |
Akodon spegazzinii sensu Pardiñas et al., 2005 : 473
| Pardinas et al. 2005: 473 |
Akodon spegazzinii sensu
| Kufner et al. 2004: 120 |
Akodon spegazzinii sensu D’Elía, 2003: 310
| D'Elia 2003: 310 |
Akodon spegazzinii sensu D’Elía et al., 2003:354
| D'Elia et al. 2003: 354 |
Akodon boliviensis sensu
| Morando & Polop 1997: 132 |
Akodon alterus sensu
| Priotto et al. 1996: 135 |
Akodon boliviensis sensu
| Polop 1991: 115 |