Murexia habbema (Tate & Archbold, 1941)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602825 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FF8D-2460-FFC7-FE42090D09C2 |
|
treatment provided by |
Felipe |
|
scientific name |
Murexia habbema |
| status |
|
39. View On
Habbema Dasyure
French: Murexie du Habbema / German: Habbema-Neuguinea-Beutelmaus / Spanish: Dasiuro del Habbema
Other common names: Habbema Antechinus
Taxonomy. Anfechinus habbema Tate & Archbold, 1941 ,
9 km N of Lake Habbema , North Slope of Mt. Wilhelmina , 2800 m, Prov. of Papua (= Irian Jaya), Indonesia.
Acceptance of the trans-Torresian distribution of Antechinus prevailed until 1984.
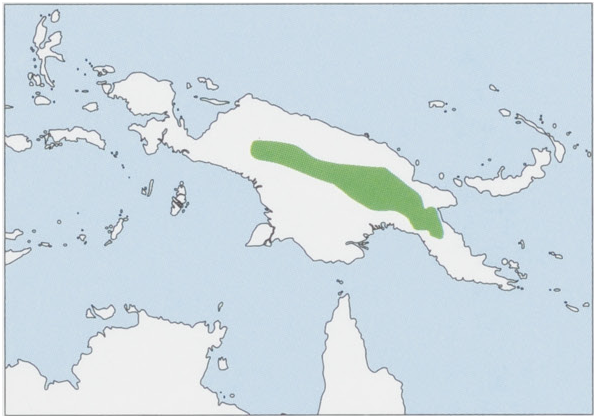
At this time, P. A. Woolley’s work on male phallic morphology indicated a dubious relationship between Australian and New Guinean members of Antechinus and thus challenged integrity of Phascogalinae. Antechinus in Australia, meanwhile, was no longer considered monophyletic, including at that time what we now regard as Dasykaluta rosamondae , Pseudantechinus macdonnellensus, P. ningbing , P. bilarni , and Parantechinus apicalis . This was followed by work clarifying species applicable to the “antechinus” of New Guinea ( melanurus , habbema, and naso ). This research indicated New Guinean species deserved generic reclassification; their inclusion in Antechinus was, as Woolley had suggested, inappropriate. DNA hybridization and albumin immunology studies confirmed the closer relationship among New Guinea species than with Australian Antechinus . Subsequent direct DNA work suggested that New Guinea taxa were sister to Australian antechinuses. Then, in 2002, S. Van Dyck’s landmark morphological study of Australian and New Guinean “antechinuses” concluded that New Guinea taxa assigned to Antechinus (pre-1984) represented three related but morphologically primitive taxa that lacked clear signs of close relationship. They were thus referred to five genera. Monotypic Micromurexia (habbema), Phascomurexia ( naso ), and Murexechinus ( melanurus ) are only distantly related to Australian antechinuses. New Guinea Murexia was thus rendered monotypic ( M. longicaudata ); morphologically, this taxon was viewed as having no especially close relationship with the more derived rothschildi , which was thus assigned to Paramurexia . Based on morphology, Australian Antechinus appeared to be monophyletic with Phascogale . Nevertheless, in the last decade additional independent DNA sequencing studies suggested that, notwithstanding distinctive morphological divergences in the group as clarified by Van Dyck, genetic differences among New Guinean species are most parsimoniously consistent with recognition of a single genus, Murexia . Several phylogenetic analyses of these sequences consistently showed strong support for monophyly of Murexia with respect to other Phascogalines, Australian genera Antechinus and Phascogale , with uncertain status of sister relationships among the three. This is now the prevailing view, so a single genus, Murexia , for New Guinean “antechinus” fauna is adopted here. Yet, parsimony merely follows the shortest path, not necessarily the “best” one. Clearly, a comprehensive revision of the entire group, incorporating genetic and morphological data and a detailed sampling of the fauna in New Guinea, is required, especially because Australian Antechinus has recently been found to harbor numerous cryptic taxa, and the most recent work indicates there are also cryptic taxa residing within at least some of the five currently recognized species of Murexia . This revision is underway. M. habbema was long considered to have been based on a mismatched skin and skull; the skin was purportedly comparable to Antechinus tafa centralis (currently M. naso ) and the skull to Antechinus wilhelmina (currently M. melanurus ). It was widely listed as a synonym of M. naso , but research by Woolley in 1989 and later an exhaustive morphological appraisal by Van Dyck indicated that it was a distinct species, distinguishable based on skull and external features. In 1952, E. M. O. Laurie described some specimens from the Tomba area in the Hagen Range at an elevation of 2500 m under the name Antechinus hageni. This has been synonymized with M. habbema, but there are still suggestions that they may be separate species. Recent work has indicated that M. habbema may represent two allopatric species; these are currently recognized as subspecies. M. habbema has a wide distribution encompassing the Central Range of the island of New Guinea (Indonesia and Papua New Guinea). Two subspecies recognized.
Subspecies and Distribution.
M.h.habbemaTate&Archbold,1941—NewGuinea,WCentralRange[Snow(=Surdiman)andStar(=Jayawijaya)Mts].
M. h. hageni Laurie, 1952 — New Guinea, E Central Range (from Mts Giluwe, Sisa, and Hagen to N Central Province in Papua New Guinea). View Figure
Descriptive notes. Head-body 11:2-12:2 cm (males) and 11-11.7 cm (females), tail 10.9-15.7 cm (males) and 11:9-14.3 cm (females); weight 28-4—45-4 g (males) and 22.7-31-2 g (females). The Habbema Dasyure is a medium-sized dasyurid that lacks stripes or spots. Its most distinctive feature is a crest of hairs running along ventral edge of tail. Ears lack post-auricular patches, and pelage is a more uniform shade throughout rather than the rufous post-auricular patches and definite warming of tones toward rump characteristic of some congeners. Claws of the Habbema Dasyure are slightly curved and slender rather than strongly curved and thick; tail is typically dorso-ventrally bicolored rather than uniform black (although sometimesit is uniform dark brown).
Habitat. Montane primary forest, mid-montane forest, beech forest, mossy forest, and subalpine grasslands at elevations of 1600-3660 m. The Habbema Dasyure occurs in disturbed primary forest, but it is not present in secondary forest habitats.
Food and Feeding. Little is known about the diet and foraging patterns of Habbema Dasyures. One study examined diet of four wild-caught species of Murexia trapped on Mount Kaindi and Mount Missim on opposite sides of the Wau Valley in the Morobe Province of Papua New Guinea. Fifty-six individuals (28 male and 26 female) were captured. Habbema Dasyures caught on Mount Kaindi produced feces that contained, by percent frequency of occurrence in feces examined, 95% beetles, 87% spiders, 50% bugs, 41% moths and butterflies, 39% grasshoppers and crickets, 37% unidentified insects, 36% worms, and 6% vertebrates (including mammal hair).
Breeding. Habbema Dasyures nest in burrow systems in the ground. One study of 18 (seven male and eleven female) wild-caught Habbema Dasyures suggested that they might breed at any time of the year. This suggestion was supported by the incidence of lactating females, often at different stages of lactation, in most months when adult females were captured, and incidence ofjuveniles in the population. The Habbema Dasyure was not successfully bred in captivity (despite two attempted pairings), but wild-conceived young were kept in captivity, although none of them survived to weaning. Males had no evidence of a sternal gland, had a scrotal width of 9-12 mm, and were estimated to mature at c.10 months. Females possessed a type one pouch containing four nipples and carried 2-4 young.
Activity patterns. There is no specific information for this species, but captive studies indicate that the Habbema Dasyure is almost exclusively nocturnal and probably largely ground dwelling.
Movements, Home range and Social organization. One study described spool-and-line tracking of the Habbema Dasyure in montane forest in the Mount Kaindi and Porgera of Papua New Guinea. Four individuals were tracked, one at Porgera and three on Mount Kaindi. Two males were tracked until the line ran out without reaching a nest, and two females were tracked to holes in the ground. These holes led to burrow systems, one of which had two entrances; both had nesting chambers located 80-100 cm below ground level. Nests were made of interwoven leaves and ferns. Both individuals returned to their burrows via indirect routes (one traversing 160 m), traveling for up to 3 m in caverns between moss, roots, and leaflitter. In one study on Mount Kaindi, 32 Habbema Dasyures were released, and eleven were recaptured at least once. Seven were recaptured twice, and two were recaptured three times. All except one were recaptured 10-100 m from points of release; the exception was a male that was recaptured six months later, some 300-400 m away.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Habbema Dasyure has a wide distribution, and no major conservation threats are known. The Habbema Dasyure occurs at high elevations in New Guinea, where it is not greatly affected by hunting or habitat loss. Montane grasslands are burned periodically, butthis is not perceived as too damaging to population numbers.
Bibliography. Armstrong et al. (1998), Flannery (1995a), Grossek et al. (2010), Helgen (2007a, 2007b), Helgen & Opiang (2011), Krajewski, Torunsky et al. (2007), Krajewski, Wroe & Westerman (2000), Krajewski, Young et al. (1997), Laurie (1952), Leary, Seri, Wright, Hamilton, Helgen, Singadan, Menzies, Allison, James, Dickman, Lunde, Aplin & Woolley (2008a), Lopez (2011), Tate (1947), Van Dyck (2002), Woolley (1984b, 1989, 2003), Woolley et al. (1991).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Murexia habbema
| Russell A. Mittermeier & Don E. Wilson 2015 |
Anfechinus habbema
| Tate & Archbold 1941 |