Antechinus leo, Van Dyck, 1980
|
publication ID |
https://doi.org/ 10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602801 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FFB4-245A-FA04-FA2F08100560 |
|
treatment provided by |
Felipe |
|
scientific name |
Antechinus leo |
| status |
|
33. View On
Cinnamon Antechinus
French: Antéchinus cannelle / German: Zimt-BreitfuRbeutelmaus / Spanish: Antequino canela
Other common names: Iron Range Antechinus, Cape York Antechinus
Taxonomy. Antechinus leo Van Dyck, 1980 View in CoL ,
Cape York Peninsula , Nesbit River , Buthen Buthen (13° 21’ S, 143° 28’ E), Queensland, Australia. GoogleMaps
The Archbold Expedition to Cape York Peninsula (1948) collected a large series of ginger-colored antechinuses from Iron Range south to Ravenshoe in Queensland (a distance of ¢.600 km through lowland to highland rainforests). G. H. H. Tate correctly recognized a close relationship between specimens from Iron Range ( A. leo ) and the more southerly A. flavipes , and thereafter referred northern specimens ( A. leo ) to A. flavipes . Unfortunately, this confusion was perpetuated in subsequent works. For example, in 1959 B. E. Horner and J. M. Taylor compared A. flavipes flavipes with A. flavipes godmani , but their sample of godmani was in fact a composite of A. godmani and A. leo . In 1962, B. Marlow referred to the Cape York form (A. leo ) as A. godmani . In 1976, R. Degabriele referred to A. leo as A. flavipes godmani . Nevertheless, during the 1976-1978 collection trip made by the Queensland Museum to north-eastern Queensland and Cape York Peninsula, a series of A. leo were collected, and it became clear they were distinctly morphologically different from A. flavipes rubeculus and A. godmani . Genetically, A. leo is profoundly different from A. godmani and, to a lesser degree, A. flavipes rubeculus. A. leo is most closely related genetically to A. adustus and A. bellus . All three species are limited to tropical distributions: A. adustus in the Wet Tropics of north-eastern Queensland, A. leo farther north in Cape York Peninsula, and A. bellus to the west in near coastal Northern Territory. A. leo and A. bellus share a curled supratragus of the external ear. Recent genetic-based phylogenies suggest that A. leo is a member of a well-supported clade that contains A. adustus , A. bellus , A. mysticus , A. argentus , and A. flavipes . Monotypic.
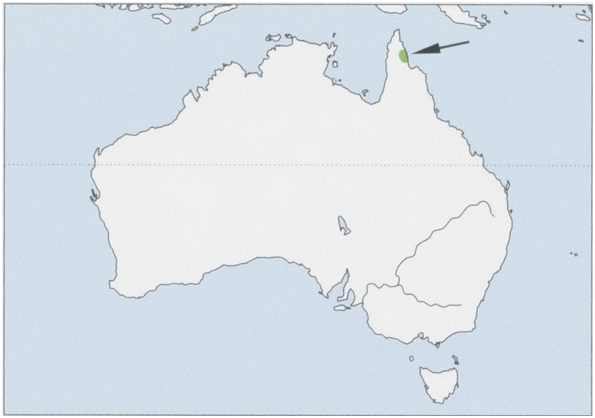
Distribution. NE Australia (N Queensland), between the Iron and Mcllwraith ranges, E Cape York Peninsula. View Figure
Descriptive notes. Head-body 14.2-16 cm (males) and 10.9-13.6 cm (females), tail 127-14 cm (males) and 8.1-12.5 cm (females); weight 67-124 g (males) and 32-74 g (females). There 1s marked sexual dimorphism for size. In museum collections, the Cinnamon Antechinus has consistently been confused with the Atherton Antechinus (A. godmani ). Both species are large for antechinuses and have an overall gingery appearance, but on closer inspection the two species are quite different in color and appearance. The Atherton Antechinus is drably and uniformly cinnamon-brown on back and rump, with orange-brown fur on sides of face and naked-looking tail. In contrast, the Cinnamon Antechinus is a more vibrant, rich cinnamon color, without obvious orange-brown fur patches on sides of face, and its tail is more evenly furred. The Yellow-footed Antechinus (A. flavipes rubeculus) is very different from the Cinnamon Antechinus, based on body color. The Yellow-footed Antechinus’s subspecies rubeculus has a grizzled grayish head changing to warm reddish-orange-toned fur on flanks, rump, and tail base; blackened tail tip; and striking pale eye ring. In fur color, the Cinnamon Antechinus most closely resembles the Fawn Antechinus (A. bellus ), but the latter is paler and grayish, sometimes with a fawn tinge on flanks and rump.
Habitat. Semi-deciduous mesophyll vine forests of fig ( Ficus racemosa , Moraceae ) and rainforest fragments along the Claudia River and Gordon Creek in the Iron Range. Type locality of the Cinnamon Antechinus receives more than 1270 mm of rain/year.
Food and Feeding. Microscopic inspection of feces of Cinnamon Antechinuses indicated that diets consists mainly of ants, beetles, caterpillars, centipedes, cockroaches, crickets, spiders, and occasionally worms. The Cinnamon Antechinus is a semi-arboreal species, foraging in microhabitats where invertebrates would be expected to be abundant. While spotlighting, a Cinnamon Antechinus was seen searching in leaflitter and crevices of a rotting log. Fourteen individuals, observed shortly after release from traps, appeared to search for invertebrates in leaf litter and crevices of logs and large trees. One radio tag was shed in litter in a dead tree trunk, in which large numbers of cockroaches and other insects were found.
Breeding. As in other antechinuses, life history of the Cinnamon Antechinus is remarkably synchronized. Mating begins about mid-September when males become very active, increasing home range sizes and extending activities into the day, presumably to search for females. When males and females meet, the larger male violently subdues her by biting the back of her neck, clasping her body, and attempting intromission. Copulation recorded in the field lasted for up to twelve minutes and probably lasts much longer. Occasionally, the female may free herself before genital locking is achieved and hide by clinging motionlessly on a branch, but the male will pursue her doggedly. Mated females regularly have patches of hair missing on their backs. Toward the end of the breeding season, males rapidly lose weight and condition; some individuals have been seen lying on the forest floor in daylight, too weak and disoriented to even take cover. By about mid-October, all males are dead. Female Cinnamon Antechinus give birth from late October to early November and carry up to ten young in their pouch. By about mid-December, young have grown too large for the mother to carry them, and she deposits them in a nest when foraging. In late January, young venture out with their mother. Juvenile males soon move away, but daughters remain in their mother’s home range and inherit her nest sites after she dies. Onset of mating in the Cinnamon Antechinus is timed such that lactation and weaning of young coincide with peak food abundance in northern Australia’s wet season. Some females live for more than two years and rear a second litter, but they rarely survive to breed a third time. In captivity, mating behavior has been observed, but females have produced no offspring. One study made detailed observations of reproductive changes in male and female Cinnamon Antechinuses. In males, developmental changes of sternal glands were synchronized each year. From February to April, sternal glands of juvenile males were covered by hair and showedlittle difference from surrounding skin, except to exude a musky smell. In May, skin of the sternal gland thickened and became pinkish. In August, the gland area lost its hair, became oily, and stained brown-red. Glands attained maximum production during the mating season; the gland became hairless, and an oily secretion smeared hair surrounding the gland. In female Cinnamon Antechinus, juvenile pouch area was covered with hair, pouch skin was pale and smooth, and nipples were barely visible. About one month before mating, a red-brown secretion accumulated around nipples, and pouch skin was stained red-brown. In the first one-half of pregnancy (early to mid-October), hair covering pouch area grew longer, and pouch area began to swell and expand. In the second one-half of the pregnancy (mid-to late October), pouch area was less covered with hair and became granular in appearance; nipples started to enlarge. A few days priorto birth, pouch area expanded to form ridges between nipples and a ridge circling nipples. Colostrum could be expressed from nipples at this stage, with gentle pressure. After birth, young attached immediately to nipples. Mammary glands of suckled nipples enlarged, whereas unoccupied nipples and their associated mammary glands regressed rapidly. Young detached from nipples in early to mid-December. Milk could be expressed from enlarged teats at this stage up until late February. Teats and pouch area regressed from this point to mid-March, but colostrum fluid could still be expressed from nipples. Pouch area then resumed prebreeding appearance (small, covered with hair, and no longer red-stained) by the end of April. Nevertheless, regressed pouch area of a parous female differed from that of a nulliparous female by having longer and paler pouch hair and slightly larger nipples.
Activity patterns. The Cinnamon Antechinus is predominantly crepuscular and nocturnal; diurnal activity is limited but increases during the mating season. In one study, two males and two females were trapped at night, were marked with radios transmitters, and released at 11:00 h the following morning. Of these, three were found in nearby traps at 15:00-16:00 h that afternoon before radio tracking commenced. All four radiotracked individuals were stationary in nest trees during radio tracking at 18:00-20:00 h. Fifteen opportunistic sightings of Cinnamon Antechinus were recorded at dusk (17:30— 19:00 h); five of these occurred during the mating season. Twenty-seven opportunistic sightings of Cinnamon Antechinus were made between 07:00 h and 17:30 h, and 23 of these were during the mating season. In the rainforests of Cape York, invertebrate activity is higher at night; this likely promotes nocturnal activity of the Cinnamon Antechinus. Potential for predation is likely the most important factor determining activity patterns. Predation risk is apparently high in daylight; in one study, Cinnamon Antechinus active during the day were attacked by potential predators (e.g. black butcherbirds, Cracticus quoyi, and variable goshawks, Accipiter novaehollandiae) and persistently mobbed by smaller birds (e.g. white-faced robins, Tregellasia leucops, and yellow-spotted honeyeaters, Meliphaga notata). Predation risk to the Cinnamon Antechinus should be accordingly lower at night because the rainforest canopy effectively disrupts moonlight, reducing visual efficiency of nocturnal predators such as owls. Overall, snakes and owls are probably the main predators of the Cinnamon Antechinus.
Movements, Home range and Social organization. Trapping results show that female Cinnamon Antechinuses stay in or near their mothers’ home ranges but sons disperse soon after leaving the nest. Of all females trapped during one study, only four had home ranges of 0-22-0-92 ha located largely within the trapping area. Home ranges of males could not be estimated because the trapping area used in the study enclosed none of them. Long-term trap records of four males and 13 females indicated considerable overlap of home ranges between and within sexes, but extent of overlap could not be estimated because home ranges of only four individuals could be measured; the remainder moved beyond detectable range outside the trapping area.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Cinnamon Antechinus is apparently common within its restricted and isolated distribution; it is at no immediate risk if its habitat remains intact. In one study, mean abundance of Cinnamon Antechinus was positively associated with density of saplings and lawyer (wait-a-while) vines. This vegetation probably provides Cinnamon Antechinuses with cover and hence reduces predation risk. Presence of small numbers (four) of Cinnamon Antechinuses in two of the three studied fragment forests may have been a result of local extinction and recolonization in these forests. Rainforest fragments were connect ed to nearby continuous rainforest (less than 1 km away) by gallery rainforests. Habitat corridors have been determined to play an important role in facilitating movement and subsequent recolonization of other species of Antechinus in fragmented habitats.
Bibliography. Baker & Van Dyck (2013c), Degabriele (1976), Horner & Taylor (1959), Leung (1999, 2008), Marlow (1962), Tate (1947 1952), Van Dyck (1980).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Antechinus leo
| Russell A. Mittermeier & Don E. Wilson 2015 |
Antechinus leo
| Van Dyck 1980 |