Antechinus godmani (Thomas, 1923)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602799 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FFB5-2459-FFC8-F3A407E804F5 |
|
treatment provided by |
Felipe |
|
scientific name |
Antechinus godmani |
| status |
|
32. View On
Atherton Antechinus
Antechinus godmani View in CoL
French: Antéchinus de Godman / German: Queensland-BreitfuRbeutelmaus / Spanish: Antequino de Godman
Taxonomy. Phascogale godmani Thomas, 1923 ,
Ravenshoe, Dinner Creek, 2900 ft. (884 m), 17° 40’ S, 145° 30’ E, Queensland, Australia.
Until relatively recently, A. godmani , a geographically restricted and poorly known species, evaded appropriate taxonomic recognition. O. Thomas suggested in his original description that A. godmani was “a large dark species of the Phascogale flavipes group.” G. H. H. Tate later cautiously attributed full specific status to A. godmani , at the same time expressing doubt regarding its distinction from A. flavipes . In 1952, after the 66" Archbold collecting Expedition to Cape York Peninsula, Tate reconsidered the identity of A. godmani and added it to a tropical “soup” of Queensland antechinuses that he called A. flavipes godmani , the ingredients of which we now know actually consisted of A. godmani , A. flavipes , and A. leo . The course of events that influenced his decision ran roughly as follows: The Archbold Expedition to Cape York Peninsula (1948) had collected a large series of ginger-colored antechinuses from Iron Range south to Ravenshoe in Queensland (a geographic range of ¢.600 km through lowland to highland rainforests). Tate correctly recognized a close relationship between specimens from Iron Range ( A. leo ) and more southerly A. flavipes , and thereafter referred the northern specimens ( A. leo ) to A. flavipes . Alongside the large, ginger Iron Range flavipes , Thomas's large, ginger godmani apparently now appeared identical, because on the basis of molar tooth row length and variable female nipple number, Tate reduced godmani to a junior subspecific synonym of A. flavipes . At the time, A. flavipes godmani consisted not only of A. leo and A. godmani but also numerous specimens of the large, reddish-colored A. flavipes rubeculus from northern Queensland. The subspecies A. f. godmani thereafter persisted in a variety of works; however, N. A. Wakefield and R. M. Warneke later once again recognized A. godmani as distinct, outlining errors with the species’ representation in earlier research. They drew attention to the unique (atleast among Australian antechinuses) tail morphology of A. godmani and dental and cranial features of the skull that separated it from all other forms. Later, a genetic phylogeny generated from allozyme loci showed A. godmani as a distinct, separate lineage but having greatest affinity with the A. swainsonii-A. minimus cluster. Relationships were resolved further using mtDNA, but although distinctive genetically, affinities of A. godmani with its congeners remained unclear. A 2002 morphological study suggested that A. godmani and A. swainsoni= A. minimus were sisters; they were linked, among a suite of skull characters, by poorly developed incisors, needle-like canines, and uncrowded premolar rows. The most recent research (2013) compared mtDNA and nDNA with morphology and found A. godmani to be clearly and deeply separated from all extant congeners (mtDNA differences of 12-9-16:3%). Indeed,this study showed A. godmani to be strongly supported as sister to a clade containing all antechinus species, except A. swainsonii and A. minimus . The morphological analysis of this study revealed A. godmani as strikingly different to all other antechinuses. Although of all antechinuses, A. leo and A. swainsonii differed significantly in the least number of morphological characters from A. godmani , there remained notable differences among the three. A. leo is generally much broader in cranio-dental skull features than A. godmani , particularly within length of the palate, and although A. swainsonii , like A. godmani , is both lean and long, A. godmani tends to be broader in a variety of measurements associated with the molar tooth row. Monotypic.
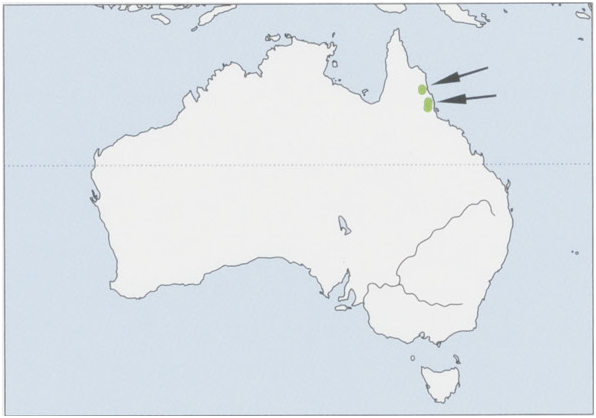
Distribution. NE Australia (NE Queensland), through highland rainforests (600-1540 m altitude) from Tinaroo Range, S of Cairns, to Girringun National Park near Mt Thorn, W of Cardwell. This distribution spans c.135 km N-S. View Figure
Descriptive notes. Head-body 10.9-15.5 cm (males) and 10.3-13 cm (females), tail 10.6-14.3 cm (males) and 9.2-11.5 cm (females); weight 62-123 g (males) and 42-67 g (females). There is marked sexual dimorphism for size. The Atherton Antechinus is distinguishable from all extant congeners based on external morphology by a combination of large size, naked-looking tail, and reddish fur on face and head. Skulls of Atherton Antechinuses are characteristically large, with a suite of long features: basicranium, palate, upper premolar tooth row, interpalatal vacuity (between holes in the palate) distance, and dentary. Two other species may co-occur with the Atherton Antechinus: the northern subspecies of the Yellow-footed Antechinus ( A. flavipes rubeculus ) and the Rusty Antechinus ( A. adustus ). These three species are easily distinguished in the field. The Atherton Antechinus is uniformly cinnamon-brown on back and rump, with orange-brown fur on sides of face, whereas the Yellow-footed Antechinus’s subspecies rubeculus has a grayish head that merges to orange-red tones on rump, flanks, and tail base; the Rusty Antechinus has uniformly dark brown fur with rusty tips on head and back and dark gray feet. Tail morphology in the Atherton Antechinus is striking and unique among Australian antechinuses. There is an abruptfinish of rump hair at base of tail, near-naked dorsal surface, more hairy ventral side, and projection of long hairs beyond terminaltip of tail. This has often been referred to as a “crest,” but length of hairs in the Atherton Antechinus is in fact no longerattail tip than that of most other Australian antechinuses;it is the near-nakedness of dorsal surface that produces the crest-like tuft. The only other antechinus-like dasyurid that duplicates tail morphology of the Atherton Antechinusis the Long-nosed Dasyure ( Murexia naso ) of New Guinea, in which the same bleaching of tail-tip hairs is often more prominent. Supratragus on the ear of the Atherton Antechinus is also unique. Structure of supratragus has been shown to distinguish the Cinnamon Antechinus ( A. leo ) from the Yellow-footed Antechinus (subspecies rubeculus). This feature also serves to separate the Atherton Antechinus from that group of antechinuses with “simple” supratragus morphology; e.g. the Rusty Antechinus, the Agile Antechinus ( A. agilis ), the Black-tailed Antechinus ( A. arktos ), the Silver-headed Antechinus ( A. argentus ), the Yellow-footed Antechinus, the Swamp Antechinus ( A. minimus ), the Bufffooted Antechinus ( A. mysticus ), the Brown Antechinus ( A. stuartii ), the Subtropical Antechinus ( A. subtropicus ), and the Dusky Antechinus (A. swainsonit). In the Atherton Antechinus, trunk of supratragus (lobe above lobe in front of earhole) and posterior distal margin are thickened. Asymmetrical thickening pulls down the naked supratragus into a flattened, squat, mushroom-shaped stump. Its structure is similar to that of the Cinnamon Antechinus and the Fawn Antechinus ( A. bellus ) but with increased corpulence. Yet it is not nearly as elaborate as the florid, finely curled and hairy structures found in such dasyurid species as the Kaluta ( Dasykaluta rosamondae ), the Fat-tailed Pseudantechinus ( Pseudantechinus macdonnellensis ), the Sandstone Pseudantechinus ( P. bilarni ), or the Ningbing Pseudantechinus ( PF. ningbing ). There is a striking similarity between supratragus morphology in the Atherton Antechinus and the equivalent structure in the Black-tailed Dasyure ( Murexia melanurus ) and the Long-nosed Dasyure, both from New Guinea. Much of the taxonomic confusion surrounding Atherton Antechinus and Cinnamon Antechinus involves their superficial external similarity. This similarity results from matching coloration of median section of each dorsal hair (non-guard). This section, in hairs taken from mid-back to rump of both species, is colored cinnamon-brown. Nevertheless, overall relative darkness of the coat of the Atherton Antechinus is attributable to the much shorter band of pigmentation, which, for hair over rump,is ¢.0-8 mm long compared to 2:3-2-5 mm in the Cinnamon Antechinus. Length of pigmentation of colorband on the Atherton Antechinus changes along back toward head such that median section of each hair over shoulders is c.1-5 mm long compared with a color band of 2 mm that is very pale silvery-yellow on the Cinnamon Antechinus. Effect and strength of color band in the Atherton Antechinus is therefore partially lost among fuscous tips and deep mouse gray basal zone of fur. Ivory yellow color band in hairs anterior to rump imparts a silvery sheen, which is characteristic of the Atherton Antechinus.
Habitat. Complex notophyll to mesophyll vine forests on basaltic krasnozems above elevations of 600 m, where rainfall is high and ground cover is open. The Atherton Antechinus is rare and apparently only patchily distributed. Floristic features ofits habitat include robust and slender woody lianas, vascular epiphytes, prominent plant buttresses, compound entire leaves, trunk spaces generally obscured by the aroid pothos, stem diameters irregular (many average 50-120 cm), and uneven canopy (averaging 21-45 m high) with emergent, mostly evergreen and umbraceous. The Atherton Antechinus does not appear to occur where the low-growing fern Cyathea rebeccae (Cyatheaceae) is common on the forest floor or in heavily disturbed forests. Female Atherton Antechinuses may require epiphytes for communal nesting when lactating; lack of epiphytes and presence of C. rebeccae indicate a high level of forest disturbance.
Food and Feeding. Atherton Antechinuses forage mostly at night. They hunt invertebrates (e.g. earthworms, spiders, moths, and cockroaches) and small vertebrates in leaf litter that has accumulated among buttress roots, around fallen logs, beneath bark of standing trees, and within epiphytes. Carrion is also eaten.
Breeding. During 3540 trap nights at Ebony Rd (near Koolmoon Creek, north-eastern Queensland), only two female Atherton Antechinuses were captured. In another study, during 5091 trap nights at Koolmoon Creek and Mount Father Clancy, just three females were captured. The pouch of a female captured on 30June 1981 contained one young (crown-rump length 6-4 mm), and a female caught on 30 October 1981 had been suckling nestling young. One female that was trapped on15July 1976 and kept in captivity gave birth later to six young; date of birth was not known, but young were first noted on 30 September 1976 and were ¢.6-5 mm long (crown-rump). Another female captured on 24July 1979 gave birth in captivity to four young on 20 August 1979. Two valuable series of females provide vital information on reproduction in the Atherton Antechinus. In six specimens collected 11-24 December 1921 near Ravenshoe, all pouches contained six nipples. In another study of four females caught in June 1989, only one displayed pouch development. In contrast, all eight females caught in July had developing or well-developed pouches. Pouch of one female caught on 14 July contained six young. In all other specimens examined, maximum nipple number was six. Male Atherton Antechinuses apparently undergo a yearly die-off characteristic of other antechinuses. Many males collected in mid-July 1967 had an eye disease, leaving them totally blind. All wild-caught males held in captivity died in July-October, and wild males could not be trapped during a late August 1978 trapping program. In a trio of captive Atherton Antechinuses (one male and two females) housed together since birth, first signs of sexual interest appeared 1-11 July, with the male investigating the cloaca of each female. The male attempted to grasp the females’ tails. At this juncture, the male’s persistence was not intense, and most encounters ended in a minor scuffle. Pouch development in these two females began on 16 September, with an increase in glandular appearance of the pouch, followed by development of heavy folds on either side of the pouch area. Neither female, however, gave birth to young—a circumstance not unusual in captive dasyurids. Thus, from this scant information, it appears that mating in the Atherton Antechinus takes place in June-July, births occur in July-August, and males die off in July-August. One study recorded no male captures after the first week of August, and pouch young were first seen in mid-August; young spent up to five weeks in the pouch and then another 8-10 weeks suckling while in a nest.
Activity patterns. The Atherton Antechinusis active during the day. It is the largest and most aggressive of the three species of antechinuses found in the wet tropics; the Rusty Antechinus and the Yellow-footed Antechinus’s subspecies rubeculus are the other two. In captivity, the Atherton Antechinus has been known to stand on its hindlegs, hissing and lunging with mouth agape when approached. When handled,it bites with gusto.
Movements, Home range and Social organization. Radio-tracking research indicates that Atherton Antechinuses use a well-defined home range of c.1 ha. Within this area, they use multiple tree hollows as retreat sites and maternal nests.
Status and Conservation. Classified as Near Threatened on The IUCN Red List. The Atherton Antechinus is only rarely encountered in scattered subpopulations that are geographically separated by human habitation and land clearing. It is locally extinct on the Evelyn Tableland as a result of anthropogenic habitat modification. Subpopulations in the area of the southern Atherton Tableland are separated by grassland and low-elevation forest. A third subpopulation of the Atherton Antechinus may occur in the upland forests of Carbine Tableland farther north, butit has yet to be found there. Trapping success is generally low, but where it does occur,it appears to be readily caught. One study estimated that prior to European settlement, total distribution of the Atherton Antechinus could have encompassed ¢.2900 km?; current distribution is estimated to be ¢.2000 km*. The Atherton Antechinusis reportedly the most endangered mammal in tropical Queensland;it is exceptionally rare and patchily distributed; widespread forest clearing near the center ofits distribution has reduced and subdivided its population; it exhibits a significant negative response to forest fragmentation; it is apparently unable to disperse across pasture and other modified habitats; and it appears to be threatened by invasions of the exotic cane toad (Rhinella marina), which have followed logging activities. The Atherton Antechinus exhibits genetic structuring overits limited distribution between the towns of Cardwell and Cairns (a distance of a mere 135 km from north to south). It is notable that the Atherton Antechinus is limited to rainforest above 600 m in elevation; its suitable habitat has most likely become fragmented over time, apparently genetically isolating proximate populations. Thus, although distribution of the Atherton Antechinus covers ¢.2000 km?, with most locations designated in national parks within the Wet Tropics of Queensland World Heritage Site, its protection by tenure may be illusory. Populations of the Atherton Antechinus have not been monitored since the 1980s to mid-1990s. Given its obligate use of highelevation rainforest, the status of the Atherton Antechinus could suffer rapid decline in the face of climate change. Its status needs to be reassessed at state and federallevels in Australia. Natural predators include moreporks (Ninox novaeseelandiae) and lesser sooty-owls (7yto multipunctata); at some roosts of lesser sooty-owls, Atherton Antechinus account for almost one-quarter of all prey taken. Domestic and feral cats also include Atherton Antechinuses in their broad list of native Australian mammal prey.
Bibliography. Baker & Van Dyck (2013c), Burnett (2008a), Degabriele (1976), Horner & Taylor (1959), Laurance (1990c, 1993), Marlow (1962), McDonald (1991), Tate (1947, 1952), Thomas (1923b), Van Dyck (1979a, 1980, 1982b, 2002), Wakefield & Warneke (1967), Watt (1991), Webb (1978).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Antechinus godmani
| Russell A. Mittermeier & Don E. Wilson 2015 |
Phascogale godmani
| Thomas 1923 |