Anlechinus minimus (E. Geoffroy Saint-Hilaire, 1803)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602805 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FFB7-245B-FAC6-FBBC0C680962 |
|
treatment provided by |
Felipe |
|
scientific name |
Anlechinus minimus |
| status |
|
34. View On
Swamp Antechinus
French: Antéchinus des marais / German: Sumpf-BreitfuRbeutelmaus / Spanish: Antequino palustre
Other common names: Little Tasmanian Marsupial Mouse
Taxonomy. Dasyurus minimus E. Geoffroy Saint-Hilaire, 1803 ,
probably Waterhouse Island, Bass Strait, Tasmania, Australia.
This species was first collected from Waterhouse Island, off the north-eastern tip of Tasmania and described by E. Geoffroy Saint-Hilaire in 1803. Three years after the description of A. swainsonii (1840) View in CoL , G. R. Waterhouse, deferring to the judgment of|. Gould, placed swainsonii View in CoL under minimus . Three years later, Waterhouse was able to examine the A. minimus holotype; he thereafter re-established swainsona, noting that “Mr. Gould imagined this species was identical with the (Antechinus) minimus of Geoffroy Saint-Hilaire; I have recently compared the two animals together, and find this is not the case. The skull of swainsonii View in CoL is proportionately narrower...” Waterhouse must have been confident indeed of his skills in external comparative assessment because the skull of the holotype of A. minimus was not removed from the mount until 1937. A. minimus was not knowingly collected from mainland Australia until 1962; however, H. H. Finlayson had encountered it in South Australia, and mistaking it for a distinct form of A. swainsonii View in CoL , named it (Antechinus) swainsonii View in CoL maritima from Port Mac-Donnell. N. A. Wakefield and R. M. Warneke later retained Finlayson’s “maritima” to describe the mainland form of A. minimus . On their assumptions that the type locality of A. minimus was Waterhouse Island and affinities of fauna on Bass Strait islands allied with the mainland rather than Tasmania, they distinguished the Tasmanian form as A. minimus minimus . Recent genetic research has shown that the two subspecies of A. minimus are sister taxa (3-4% divergent at mtDNA), and A. minimus is most closely related to A. swainsonii View in CoL . Two subspecies recognized.
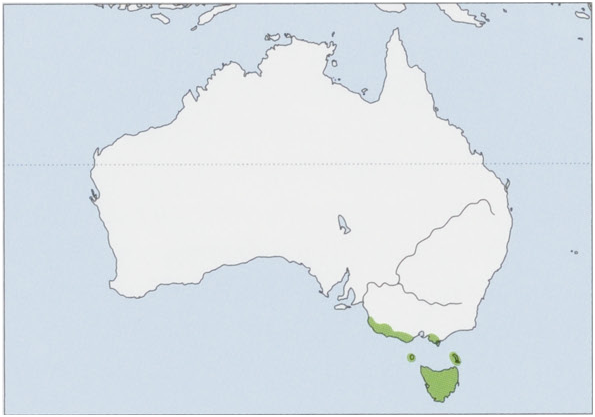
Subspecies and Distribution.
A.m.minimusE.GeoffroySaint-Hilaire,1803—TasmaniaandtheBassStraitIs.
A. m. maritimus Finlayson, 1958 — SE Australia, in S Victoria (W from S Gippsland and Wilson’s Promontory and nearby Sunday, Kanowna, and Great Glennie Is) W to SE South Australia (to Robe). View Figure
Descriptive notes. Head—body 10.3-14 cm (males) and 9.8-11.7 cm (females), tail 6-5—10 cm (males) and 6.7-8.5 cm (females); weight 50-129 g (males) and 36-68 g (females). There is marked sexual dimorphism for size. Swamp Antechinuses are leaden gray on head and shoulders, grading into rich yellowish-brown on rump and flanks; belly fur is grayish-yellow or buff. Tail has short hair, grizzled dark brown above and paler below. Fur is coarse and grizzled; fore claws are long. Eyes and ears are small. Compared with congeners, Swamp Antechinuses are most similar in coloring to the Yellow-footed Antechinus (A. flavipes ). In contrast to the Swamp Antechinus, the Yellow-footed Antechinus has a marked pale eye ring, more orange-toned rump, fur on feet and tail base, and a more darkened tail tip. Tail length is proportionately closer to head-body length in the Yellow-footed Antechinus compared with the Swamp Antechinus.
Habitat. Damp habitats with a high percent cover of understory vegetation in forest, woodland, heathland, tussock grassland, and sedgeland. The Swamp Antechinus prefers low-elevation sites with a southerly aspect and gentle slope. It is often found close to drainage lines and swamps.
Food and Feeding. The diet of Swamp Antechinuses on Kanowna Island included a wide variety of prey. Remains of insect larvae, beetles, spiders, flies, and ants were frequently identified in feces of trapped individuals; centipedes, scorpions, grasshoppers, and lizards were also occasionally found in feces. Similarly, various arthropods were found in feces of Swamp Antechinus from the Otway Ranges and Great Glennie Island. This large variety of prey items would suggest that the Swamp Antechinusis a generalist. The study on Kanowna Island found a high incidence of moth larvae in the diet. Moth larvae remains were found in ¢.95% of feces in August-October. Although frequency of moth prey itemsfell in November and January, larvae were still the most important prey item in the diet, in terms of number, bulk, and frequency.
Breeding. Breeding of Swamp Antechinuses occurs in May-August and,as is the case for congeners, is normally synchronized within populations, but it varies across the distribution. Males die-off after breeding, but some females survive to breed in a second or third year. Following gestation (typically 28-32 days) and birth, young remain attached to teats for c¢.2 months. They remain in a leafy nest while the mother forages, emerging from the nest at ¢.3 months old. Litter size at weaning usually equals number of teats in the mother: eight on the mainland (subspecies maritimus) and six in Tasmania (nominate subspecies minimus ). Gestation varies with different populations; it lasted 2-3 weeks in a population at Anglesea, one month on Great Glennie Island, and more than six weeks on Kanowna Island. This suggests either variable duration of mating or sperm storage. A large variation in size of pouch young was recorded in a high-density population on Kanowna Island, suggesting lower synchronization of timing of births compared with other populations of antechinuses. Precise causes of synchrony have not been established, but it may at least in part be associated with availability of food resources.
Activity patterns. Swamp Antechinuses are mostly active in the first few hours after dark and before dawn, with some daytime activity.
Movements, Home range and Social organization. In the eastern Otway Ranges of Victoria, dispersal of nine litters of pouch young (n = 62) was assessed following two breeding seasons. Young males remained on the natal site until December—January and dispersed before the breeding season. New males entered the population in January=June. More than 50% of females were residents at the study site and remained there to breed; remaining females were trapped a single time. After the male die-off, movements of pregnant females increased, and they appeared to expand their home ranges. Female Swamp Antechinuses are philopatric, and males disperse, as observed in other species of Antechinus . Nevertheless, most antechinuses disperse abruptly after weaning, but Swamp Antechinuses were dispersed 2-3 months after weaning.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Preferred habitat of Swamp Antechinusesis limited, so they are patchily distributed and thus sensitive to human disturbance, particularly land clearing and urban development. The Swamp Antechinus prefers late successional vegetation. Some populations were eliminated by bushfire in the eastern Otway Ranges, Victoria, and have unfortunately taken 20 years to re-establish. Current conservation threats are habitat and population fragmentation, drainage of swamp habitat, and frequentfire. Peak densities in the eastern Otways area and Walkerville in south Gippsland were much lower than on islands off Wilsons Promontory, such as Great Glennie Island and Kanowna Island where astonishing densities of up to 50 ind/ha have been recorded. Such high densities on islands are well known among small mammals and have mainly been attributed to lower interspecific competition and predation than experienced by mainland populations.
Bibliography. Allison et al. (2006), Baker & Van Dyck (2013a, 2013b, 2013c), Finlayson (1958a), Geoffroy Saint-Hilaire (1803), Gibson etal. (2004), Magnusdottir et al. (2008), Sale et al. (2006), Van Dyck (1997), Wainer (1976), Wakefield & Warneke (1963), Waterhouse (1840, 1846b), Wilson (1986), Wilson & Bachmann (2008), Wilson, Aberton & Reichl (2001), Wilson, Bourne & Jessop (1986).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.