Lycaena ratushinskayae, Churkin & Kolesnichenko, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4555.4.6 |
|
publication LSID |
lsid:zoobank.org:pub:1A97A102-00B3-47B5-BF43-CBE028FC81E7 |
|
DOI |
https://doi.org/10.5281/zenodo.5932854 |
|
persistent identifier |
https://treatment.plazi.org/id/8454AF10-D6E2-41AE-840F-25D7D9AC16C9 |
|
taxon LSID |
lsid:zoobank.org:act:8454AF10-D6E2-41AE-840F-25D7D9AC16C9 |
|
treatment provided by |
Plazi |
|
scientific name |
Lycaena ratushinskayae |
| status |
sp. nov. |
Lycaena ratushinskayae sp. n.
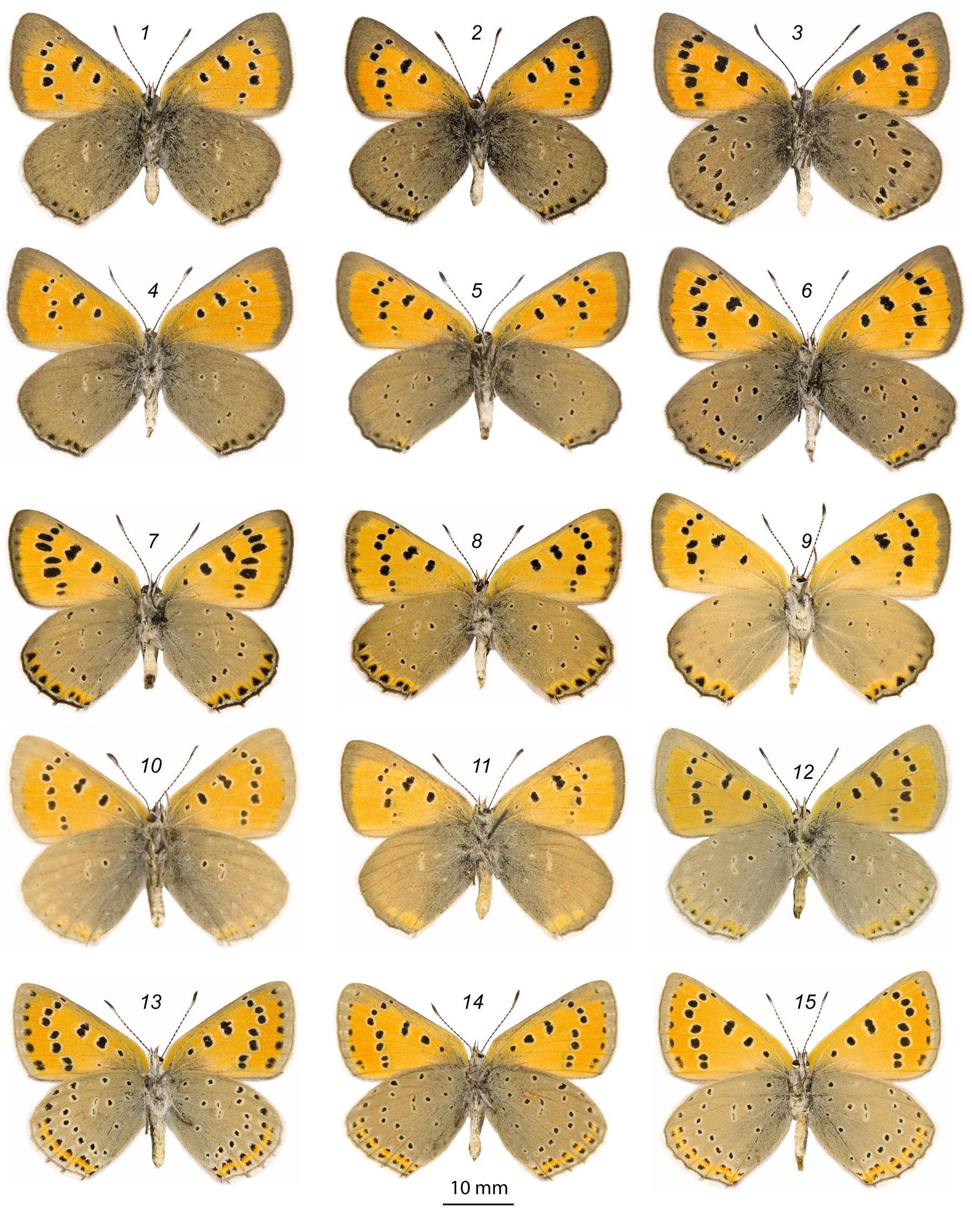
Plate 1 View PLATE 1 and 2 View PLATE 2 , figs. 1 (holotype), 2–6 (paratypes).
Material. Holotype: GoogleMaps ♂, SW Mongolia, Khovd aimak, Baitag Mts., Buduun Khargaityn GoogleMaps r., 2600–2800m, 2– 3.07.2018, 45°13' N, 90°56' E, S. Churkin leg. (SDM).
Paratypes: 34 ♂, 25 ♀, same data, S . Churkin, K. Kolesnichenko leg. ; 2 ♂, 1 ♀, same data, 2400 m.a.s.l., V. Pletnev, Odbayar Tz. leg. ( paratypes are in the private collections of S . Churkin (Reutov), V . Pletnev (Reutov), K . Kolesnichenko (Moscow), Odbayar Tz. (Ulan-Bator), P . Beda (Ljubertzy), G . Grieshuber (Ortenburg, Germany), G . Bozano (Milan, Italy) as well as the collection of the Institute of General and Experimental Biology MAS .
Notes. The Buduun Khargaityn River is named as “Buruun” on some maps and Internet resources, which is certainly wrong. The true name is translated from Mongolian as “the river of the thick larches”. The correct spelling of the range name should be “Baaitag”, however, we used simply “Baitag” according to the actual traditions of spelling Mongolian names.
Description. Male. Holotype forewing length is 17.5 mm, paratypes 15–18.5 mm.
Antennae: black rings wider than whitish rings at the segments.
Upperside ground colour saturated orange-red. Fringes white.
Forewing upperside with 2 black discal spots, 8 postdiscal spots and a black margin. As a rule, this pattern is full, spots sometimes enlarged, rarely slightly or partly reduced. Black margins are very thick so that they fully include the submarginal row of blackish spots; as a result, these spots are not obvious at all (or barely obvious). Black margins practically not narrowed towards the anal angle of the forewings.
Hindwing upperside has one linear discal spot, postdiscal row is more or less reduced (especially from the costal side) and usually consists of 4–5 spots. Submarginal black spots large and merged with the margin; submarginal orange band absent.
Forewings underside has widened blackish-gray margins, the yellowish orange discal area is reduced especially at the apex. The colour of the discal area definitely differs from the upperside ground colour. Postdiscal black spots with obvious whitish rings. Submarginal row of black spots is absent.
Hindwings underside with unusual dark (even blackish) gray ground colour. Black pattern usually reduced so that only 4–6 small black dots are developed in the postdiscal row. Rarely the pattern is full (8 or even 9 spots), very rarely all spots enlarged (Pl. 2: 3). Submarginal orange band absent, only some orange lunules weakly expressed at the anal angle. Usually 2–4 unclear submarginal black dots developed at the anal and cubital area. Discal spot is weak and usually looks like two thin lines with lightened aureole.
Female. FW length is 18–21 mm.
The main colouration is the same as in males, but ground colour sometimes lighter being orange or even yellowish-orange. The discal yellowish area at the forewing underside not reduced from the apex. All other characters of males are presented; hindwings underside with statistically more developed black pattern while the ground colour is not lighter.
Male genitalia. Genitalia is large and strongly sclerotized. Arms of uncus are moderately long having nearly the same width everywhere, falx long with sharp claws and curved at the middle with an angle of 90 degrees (Pl. 3: 1, 2). Valva is massive, with oval and long proximal part, distal part is short and bears a row of strong teeth at the margin (Pl. 3: 3, 4). The size and even the row of the teeth of each valvae are slightly different. Teeth are also not uniform and sometimes look broken or angled. Juxta is strongly developed and consists of a strong “leg”, two flattened plates, each plate bears the long and strong curved fang at the external side and a small additional inner tooth (Pl. 5: 7, 8, 9). The “leg” is joined with the proximal parts of the valvae. Aedeagus is straight with a curved proximal part and one cornutus in the vesica (Pl. 3: 5).
Individual variation and forms. One female has very large submarginal spots on the underside as well as an extensively developed black pattern on the lightened upperside being moderately similar to the female of L. dabrerai . However, the black margins are thick while the underside is really dark-gray.
Another female has a fully developed forewing pattern but a strongly reduced hindwing pattern (only 2 small black postdiscal spots are obvious at the upperside).
Two females have a hindwing underside with distinctive dark but not dense suffusion which also includes some violet scales, as a result the wings bear a violet gleam.
Diagnosis. 1. External differences. Male upperside has the deepest reddish colour (very different from yellowish L. dabrerai and yellowish-orange L. odbayar ) similar to the darkest sample of L. violacea . It differs from L. violacea by an undeveloped submarginal pattern on the hindwing (both on the upperside and underside; the submarginal orange band is reduced), monotonous darkened underside and thick marginal black bands on the forewings with straight inner margin.
The latter character is strongly specific: in all other species the black margins are narrow and, moreover, become narrower at the anal angle. Thus, the inner border of the band is wavy (undulating) because the submarginal row of small black spots (joined with this thin marginal band) is obvious. Only the females of L. dabrerai have more or less wide marginal forewing bands, but they are always wavy, and the uppserside is always clearly yellowish.
The dark gray (and even slightly blackish) underside also seems to be specific. The closest L. dabrerai has a distinctive brownish underside (not blackish but rather with an olive hue), females are always lighter than males. The new species has a much darker underside without expressed sexual dimorphism. On the other hand, L. violacea from Gurvan-Saikhan also has a very dark underside—but it looks abruptly different from the new species because all the submarginal pattern (i.e. orange and black spots) is contrasting and developed.
The strongly developed blackish pigment at the underside and forewing margins contradicts the reduction of the submarginal black spots at both wings, such a situation can only be the result of genetically based differences.
The degree of pattern reduction is very variable in L. dabrerai and L. ratushinskayae . L. odbayar has the most reduced underside pattern: only in few specimens this pattern is more or less obvious among tens of paratypes and topotypes, the butterflies from Mongolian Altai (Sutai Uul area) have even more reduced black spots.
On the contrary, L. violacea has fully developed black and orange submarginal patterns; the butterflies with reduced submarginal spots are very rare and certainly present aberrations. Another rare aberration presents specimens with a reduced postdiscal row on the hindwing upperside—this tendency is known for all taxa of the group, including new species.
2. Genitalia distinctions. As it was stated previously, juxta presents the main specific characters within this group of species ( Churkin, 2004). The first author applied a term “tooth” to the strongly sclerotized, stretched spike, located at the distal ending of the juxta. This term seems not so convenient because valvae also have a number of “teeth”. Thus, we replace “tooth” of the juxta with the term “fang”.
Males of the new species have the strongest and longest fangs which gradually curve from the wide basal part to sharpened ends; the fangs are not strongly widened at the base. Such a combination of characters abruptly differs from all related species (see also in Discussion). It might resemble the fangs of L. splendens being much longer and heavier, but in reality they are obviously steeply bent and are not directed inwards.
In addition, the small teeth at the distal ends of the valva are larger than in L. violacea (this character is only slightly obvious in the photo because the teeth are curved down inwards), the longer uncus do not have basal widening (the latter is typical for L. violacea ), aedeagus has a small protrusion from the ventral side of the distal part (obvious at the basal end of the part).
Bionomics (Pl. 6: 1–3). Inhabits steep dry slopes at high altitudes. Very local. The food plant (Pl. 6: 3) is similar to Rheum compactum L. (= altaicum Losinsk.), but it requires in final confirmation. It is worth noticing that Rheum nanum Siev. ex Pall. —the food plant of L. dabrerai— is numerous at the margins of the desert in proximity to the Baitag Range (especially at the eastern part of the range). We did not collect the butterflies, but they must be there for sure because this area is situated inside of the known area of L. dabrerai . A photo of this Rheum is shown in Plate 6 View PLATE 6. 1 (fig. 4), where the leaves are damaged in a manner typical for the larvae of Lycaena .
Distribution (Pl. 7). Known only from the type locality. The new species is completely isolated and widely separated from all the known populations of Lycaena , except for L. dabrerai (see above).
In the map, we recorded all the known localities for all the taxa in study in Mongolia according to our data as well as literature ( Korshunov & Soljanikov, 1976; Korshunov, 1977; Tshikolovets et al., 2009).
L. dabrerai lives in the deserts and deserted foothills of Transaltai and Dzhungarian Gobi. The distribution area of L. violacea is very large and covers grassy biotopes (dry steppificated meadows, steppificated river valleys, etc.) from Dahuria to Tuva and Altai (where it is quite rare), including all northern Mongolia and the Khangai range. An isolated population was found in the Gurvan-Saikhan Mts. ( Churkin, 2004) while information from other ranges of Gobi Altai is not available. L. odbayar occurs at the southern limits of Mongolian Altai—from Gichgenyi Nuruu (type locality) to Sutai Uul, the biotope is alpine meadows (the same as in the new species).
Notes. The maps published by Tshikolovets have some mistakes with locality points. It is particularly important that the localities for L. dabrerai from Mongolian Altai were not confirmed further ( Yakovlev, 2012) as well as some points for L. violacea are simply misplaced. For unknown reasons both Tshikolovets and Yakovlev used the name “ adbayar ” instead of odbayar, Yakovlev marked “Khan-Tajshiryn-Nuruu” as the type locality for this species ( Yakovlev, 2012: 67).
Etymology. The species is named after Irina Ratushinskaya (1954–2017), a great Russian poet and writer.
| S |
Department of Botany, Swedish Museum of Natural History |
| K |
Royal Botanic Gardens |
| V |
Royal British Columbia Museum - Herbarium |
| P |
Museum National d' Histoire Naturelle, Paris (MNHN) - Vascular Plants |
| G |
Conservatoire et Jardin botaniques de la Ville de Genève |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |