Cliona minuscula, Schönberg, Christine Hanna Lydia, Grass, Stefanie & Heiermann, Anke Tarja, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.173882 |
|
DOI |
https://doi.org/10.5281/zenodo.5617022 |
|
persistent identifier |
https://treatment.plazi.org/id/F003CC35-FFA6-5739-FEBF-E14AFAABFDFA |
|
treatment provided by |
Plazi |
|
scientific name |
Cliona minuscula |
| status |
sp. nov. |
Cliona minuscula , sp. nov.
Material examined
Two specimens of Cliona minuscula , sp. nov. from Little Pioneer Bay, Orpheus Island, Palm Island Group, Central Great Barrier Reef, Australia (18 40’S 146 30’E; Fig. 1 View FIGURE 1 ):
Cliona minuscula , sp. nov. holotype: QM G322595, wet sample in alcohol, original sample number: 5.46, 27.10.2003: in dead, unattached shell of Tridacna crocea in 1.0 m depth on reef crest ( Fig. 2 A–B).
Cliona minuscula , sp. nov. paratype: QM G322596, slide preparation, entire specimen was used up for the preparation, original sample number: 2.80, 25.10.2003: in a dead brain coral in 2.2 m depth, from the reef flat.
Other species examined:
Cliona caesia new comb., various specimens from Little Pioneer Bay, Orpheus Island, Palm Island Group, Central Great Barrier Reef and Heron Island, Capricorn Bunker Group, Southern Great Barrier Reef Australia, wet samples in alcohol, Queensland Museum, spicule preparations in first author’s collection.
Cliona patera : MHNG38767 from the Museum in Geneva, dry specimen, section of a massive sponge. Sample site unknown.
Description of the holotype QM G322595
Live ectosome dark brown, endosome lighter brown to beige. Colour fading when frozen or in alcohol, with endosomal tissue becoming almost transparent. Papillae minute, mean papillar diameter 0.44 mm when contracted (minimum = 0.20 mm, maximum = 0.70 mm, SD = 0.14 mm, N = 25). Form circular in outline, occasionally irregular. Contracted papilla margins flush with surface, but towards centre ‘sunken’ into substrate, latter forming small circular pits around papillae ( Fig. 2 C–D). Some of these pits in sample QM G322595 no longer filled with sponge tissue, but with mud and algae. Papillae evenly dispersed, mean distance from each other 0.40 mm (minimum = 0.16 mm, maximum = 0.80 mm, SD = 0.18 mm, N = 25), fusion rare. Except for small areas opposite shell umbo, tissue not traversing entire width of shell, but penetrating to a depth of 1–2 mm. Sponge tissue emerging on inner side of shell forming netlike pattern ( Fig. 2 B). External erosion traces on shell outer surface as papillar pits and shallow grooves that connect papillar pits in close vicinity to each other ( Fig. 2 D). Endolithic erosion chambers minute, appearing finely porous, with spherical to oval chambers ( Fig. 2 E–F). Under magnification some chambers more irregular in shape as result of merging ( Fig. 2 F). Mean chamber diameter 0.59 mm (minimum = 0.2 mm, maximum = 1.3 mm, SD = 0.29, N = 25), mean diameter of canals connecting chambers 0.20 mm (minimum = 0.08 mm, maximum = 0.33 mm, SD = 0.07, N = 10).
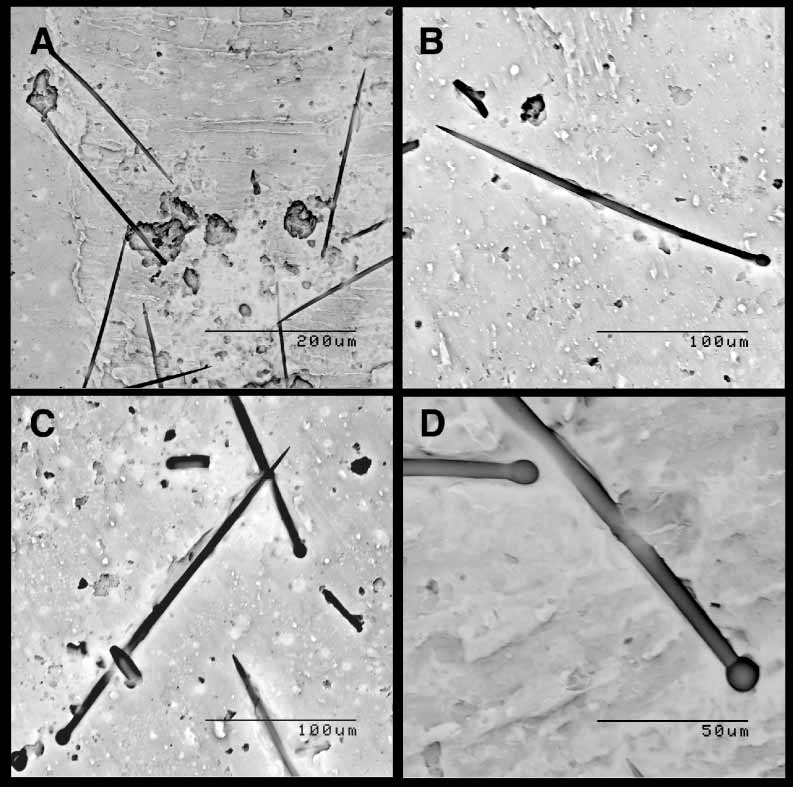
Endosomal tissue thinly coating chambers and forming fragile membranes. Tylostyles in papillae in loose palisade, spicule points protruding from tissue by about 1/3 of spicule length ( Fig. 2 G). Tissue with zooxanthellae (dinoflagellate symbionts) concentrated at the sponge surface ( Fig. 2 H). Endosomal tylostyles aligned in parallel, forming bundles. Bundles in confused orientation. Skeleton comprised of megascleres only, i.e. of tylostyles ( Fig. 3 View FIGURE 3 ). Mean tylostyle dimensions comparatively small, with shaft length x shaft width x tyle width: 225.3 x 4.5 x 6.8 µm ( Tab. 1). Tylostyle width sizefrequency distributions skewed to left, indicating very uniform final size. Most tyles oval, slightly elongated ( Fig. 3 View FIGURE 3 A–B), but some round ( Fig. 3 View FIGURE 3 C–D), rarely with secondary swellings. Shafts predominantly straight ( Fig. 3 View FIGURE 3 A–B), neck regions usually subtle, few with sharp creases ( Fig. 3 View FIGURE 3 C–D). Axial threads extending about 2/3 into tyles. No vesicles observed when using light microscopy, but occasionally striations in tyles (layering of silica).
Additional species in the holotype
Specimen QM G322595 contains three other species of bioeroding sponges: Cliona ensifera , Cliona cf. orientalis in form and an unidentified species of Aka . Latter two species were only tentatively or incompletely identified, as C. cf. orientalis lacked microscleres and Aka sp. is a small specimen with indistinct fistules. Aka sp. and C. minuscula , sp. nov. occur in two tiers, with C. minuscule , sp. nov. forming the upper layer near the shell surface, being traversed by Aka sp. papillar canals ( Fig. 2 E–F). The additional species in QM G322595 are distinct from C. minuscula , sp. nov. by their spicules ( Tab. 2, Fig. 4), papillar size ( Tab. 2, Fig. 2 A) and form of erosion chambers ( Tab. 2, Fig. 2 B, E–F).
Ecology and distribution
To date only two specimens of Cliona minuscula , sp. nov. have been found, and only from one sample site at Orpheus Island, Palm Island Group, Central Great Barrier Reef.
The sponges were sampled from very shallow depths between 1 and 2 m in unattached rubble (dead clam shell) and in part of the reef framework (dead massive coral). The specimens were from the reef flat, i.e. from the mixed zone ( Schönberg 2001), and from the reef crest.
Etymology
The species name ‘ minuscula ’ was chosen, as compared to other species of Cliona , the new species has minute papillae and erosion chambers and smaller than average tylostyles.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.