Sciopemyia sordellii ( Shannon & Del Ponte, 1927 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5195.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:54F044B5-E7CC-44F1-B1F2-CF3A717289B9 |
|
DOI |
https://doi.org/10.5281/zenodo.7198732 |
|
persistent identifier |
https://treatment.plazi.org/id/F00D87CA-B27A-3730-FF1A-61ABFB82F924 |
|
treatment provided by |
Plazi |
|
scientific name |
Sciopemyia sordellii ( Shannon & Del Ponte, 1927 ) |
| status |
|
Sciopemyia sordellii ( Shannon & Del Ponte, 1927) View in CoL
( Figs 1–4 View FIGURE View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Phlebotomus sordellii Shannon & Del Ponte, 1927: 730 View in CoL ( ♂, Resistencia , Chaco, Argentina).
Flebotomus nordestinus Mangabeira, 1942: 327 View in CoL ( ♂, Nova Olinda, Ceará, Brazil); Young & Morales 1987: 662 (as synonym of Ph. sordellii View in CoL ).
Phlebotomus longicornutus Floch & Abonnenc, 1943: 6 View in CoL ( ♂, ♀, Montabo, Cayenne, French Guiana); Barretto 1946: 534 (as synonym of F. nordestinus View in CoL ); Forattini 1960: 478 ( Amapá, Brazil).
Lutzomyia nordestina ; Barretto 1962: 96 (list.); Llanos 1973: 32 ( ♂, ♀, figs., dist., Loreto; Madre de Dios; Peru) ; Martins et al. 1978: 165 (refs., dist.); Mayrink et al. 1979: 131 (dist., Minas Gerais, Brazil) ; Young 1979: 233 (figs., refs., dist.); Fraiha et al. 1980: 21 (dist., Loreto, Peru) ; Morales & Minter 1981: 97 (dist., Caqueta, Colombia) ; Biancardi et al. 1982: 168 (dist., Rondônia, Brazil) ; Arias & Freitas 1982: 404 (dist., Acre, Brazil) ; Ready et al. 1983: 780 ( Pará , Brazil) ; Young & Rogers 1984: 599 (list., Cañar; Guayas; Los Rios; Napo; Pichincha; Ecuador) ; Ryan 1986: 120 ( ♂, ♀, figs., Pará , Brazil) ; Ryan et al. 1987: 356 (nat. trypanosome infection, Pará , Brazil) ; Feliciangeli 1988: 110 (list., Venezuela) ; Feliciangeli et al. 1988: 48 (dist., Amazonas, Venezuela) ; Le Pont & Desjeux 1992: 266 ( cf. to vattierae View in CoL ).
Lutzomyia sordellii View in CoL ; Theodor 1965: 187 (listed); Young & Morales 1986: 662 (figs. of lectotype); Alexander et al. 1992: 125 (rec., Cañar; Guayas; Los Rios; Pichincha; Sucumbios; Ecuador); Young & Duncan 1994: 54 ( ♂, ♀, figs., list., dist., tax., identification key); Andrade-Filho et al. 1997: 13 (dist., Minas Gerais, Brazil); Galati et al. 1997: 31 (dist., Mato Grosso do Sul, Brasil); Barros et al. 2000: 16 (dist., Maranhão, Brazil); Andrade-Filho et al. 2001: 96 (list., Piauí, Brazil); Oliveira et al. 2003: 19 (dist., Mato Grosso do Sul, Brazil); Ferreira Silva & Vasconcelos 2005: 38 (dist., Pernambuco, Brazil); Margonari et al. 2010: 47 (dist., natural infection by Leishmania View in CoL , Minas Gerais, Brazil); Rebêlo et al. 2010: 26 (list., Maranhão, Brazil); Alencar et al. 2011: 118 (ecology, Amazonas, Brazil); Azevedo et al. 2011: 40 (dist., Maranhão, Brazil); Oliveira et al. 2011: 106 (dist., Pará, Brazil); Guimarães et al. 2012: 45 (dist., Pernambuco, Brazil); Queiroz et al. 2012: 45 (list., Mato Grosso, Brazil); Kent et al. 2013: 6 (dist., Sabajo Hills, Republic of Suriname); Nascimento et al. 2013: 125 (list., Minas Gerais, Brazil); Guimarães et al. 2014: 56 (molecular detection of Leishmania View in CoL ); Miranda et al. 2015: 146 (dist., Pernambuco, Brazil); Pereira Filho et al. 2015: 8 (dist., Maranhão, Brazil); Chagas et al. 2016: 7 (dist., Pará, Brazil); Pinheiro et al. 2016 (dist., Rio Grande do Norte, Brazil); Guimarães & Silva et al. 2017 (food source, molecular detection of Leishmania View in CoL ); Silva et al. 2017: 26 (dist., molecular detection of Leishmania View in CoL , Maranhão, Brazil); Pereira Filho et al. 2018 (molecular detection of Leishmania View in CoL ); Thies et al. 2018: 177 (natural infection by Leishmania hertigi ).
Psychodopygus nordestinus ; Forattini 1973: 475 ( ♂, ♀, figs., tax.).
Sciopemyia sordellii View in CoL ; Galati 2003: 44 ( ♂, ♀, list., figs., dist., tax., identification key); Galati et al. 2003: 47 (dist., Mato Grosso do Sul, Brazil); Bejarano et al. 2006: 26 (tax.); Oliveira et al. 2006: 101 (dist., Mato Grosso do Sul, Brazil); Shimabukuro et al. 2007: 51 ( ♂, ♀, figs., tax.); Andrade-Filho et al. 2008: 37 (ecology); Cutolo et al. 2009: 18 ( ♂, figs., tax.); Dorval et al. 2009: 104 (dist., Mato Grosso do Sul, Brazil); Dorval et al. 2010: 43 (dist., Mato Grosso do Sul, Brazil); Galati et al. 2010: 54 (dist., São Paulo, Brazil); Salómon et al. 2010: 69 (dist., Misiones, Argentina); Vilela et al. 2011: 6 (dist., Tocantins, Brazil); Fordellone et al. 2012: 45 (dist., Paraná, Brazil); Machado et al. 2012: 107 (dist., Tocantins, Brazil); Carvalho et al. 2013: 8 (ecology); Cutolo et al. 2013: 19 (dist., São Paulo, Brazil); Pinheiro et al. 2013: 38 (dist., Rio Grande do Norte); Brilhante et al. 2015: 57 (dist., Mato Grosso do Sul, Brazil); Pinto et al. 2015: 10 (DNA barcoding); Rêgo et al. 2015: 10 (molecular detection of Leishmania View in CoL ); Sanguinette et al. 2015: 8 (dist., Minas Gerais, Brazil); Saraiva et al. 2015: 10 (dist., Minas Gerais, Brazil); Dorval et al. 2016: 23 (dist., Mato Grosso do Sul, Brazil); Oliveira et al. 2016: 11 (dist., Mato Grosso do Sul, Brazil); Peres Dias et al. 2016: 58 (dist., Rio de Janeiro, Brazil); Pinheiro et al. 2016: 9 (dist., Rio Grande do Norte); Shimabukuro et al. 2016 (dist., Bahia; São Paulo; Brazil); Carvalho et al. 2016 (molecular detection of Leishmania View in CoL ); Slezag et al. 2017 (dist., Chaco Province, Argentina); Silva et al. 2017 (dist., Pernambuco, Brazil); Tonelli et al. 2017: 12 (dist., Minas Gerais, Brazil); Ávila et al. 2018: 14 (natural infection by Leishmania View in CoL ); Lana et al. 2018: 13 (dist., Minas Gerais, Brazil); Rodrigues et al. 2018 (DNA barcoding); Barrios et al. 2019: 14 (dist., Mato Grosso do Sul, Brazil); Costa et al. 2019: 52 (dist., Minas Gerais, Brazil); Pereira Júnior et al. 2019: 114 (dist., Rondônia, Brazil); Santos et al. 2019: 14 (dist., molecular detection of Leishmania View in CoL , Amapá, Brazil); Da Silva et al. 2020 (molecular detection of Leishmania View in CoL ); Torchitte et al. 2020 (dist., food source, Rondônia, Brazil); Costa et al. 2021 (bloodmeal identification).
Diagnosis. Female: preapical papilla on flagellomere III present posterior teeth slanted, facing the central region of cibarium; anterior teeth arranged in a transverse row; spermathecae also transversally striated, though without the tubular aspect, clearly wider than the individual spermathecal ducts and with sessile terminal knob. Male: preapical papilla on flagellomere III present; gonocoxite without basal tuft of setae; flagellomere I ≥ 405 µm; aedeagal ducts ≤ 440 µm; epandrial lobe ≤ 157 µm; presence of one apical spine and upper external spine at the subapical level in the gonostyle.
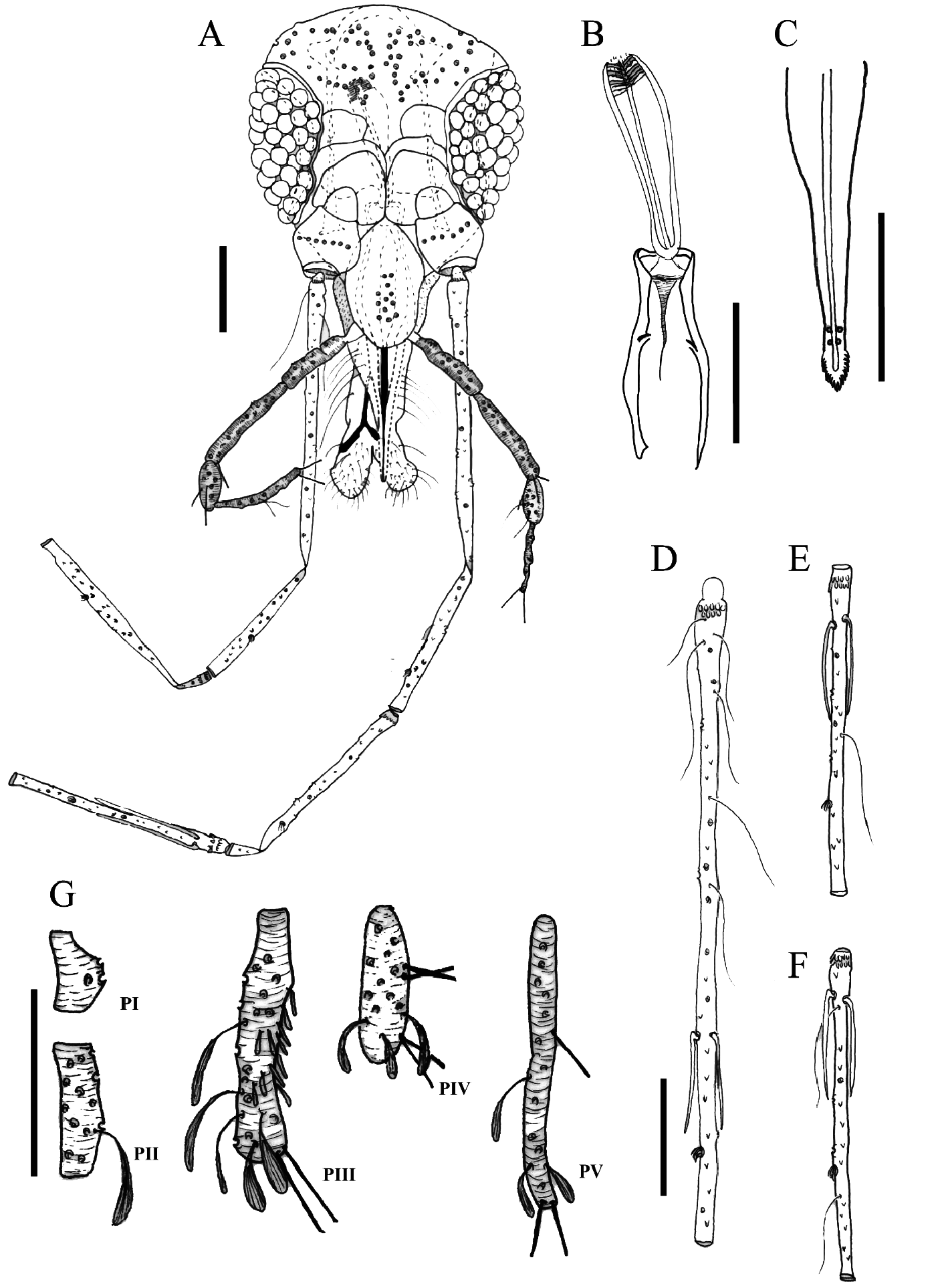
Female. Head ( Fig. 1A View FIGURE ): 367 (363/381, n = 2) in length, 328 (330/339, n = 2) in width. Arrangement of deciduous bristles in the occiput region forming an “X” shape; clypeus 122 (116/136, n = 2) in length; eyes 177 (177/180, n = 3) in length, 99 (96/99, n = 2) in width; interocular distance 148 (133/151, n = 2). Pharynx with small teeth ( Fig. 1B View FIGURE ). Cibarium ( Fig. 1B View FIGURE ) with four posterior teeth well developed, curved towards the central region of the cibarium, eight anterior teeth distributed in a transverse row and eight lateral teeth flanking the posterior teeth; sclerotized area short and triangular; sclerotized arch incomplete. Labrum-epipharynx ( LE) ( Fig. 1C View FIGURE ): 174 (162/168, n = 2); mandible as shown ( Fig. 1D View FIGURE ). Apical region of hypopharynx with 18 teeth ( Fig. 1F View FIGURE ). Lacinia of maxilla with 11–12 external teeth and 16 internal teeth ( Fig. 1E View FIGURE ). Antenna ( Figs 1G–I View FIGURE , 2A–K View FIGURE 2 ): flagellomere length: FI 470 (470/493, n = 2); FII 237 (228/242, n = 2); FIII 209 (228/251, n = 2); FXIII 96 (78/110, n = 2) and FXIV 67 (67/72, n = 2). Ascoids: short posterior spur with a peduncle type of insertion and the anterior spur is long in FI and reaches the level of the preapical papilla ( Fig. 1G View FIGURE ); the external ascoids implanted more apical than the internal ones, in FI; presence of preapical papilla on FI– FIII ( Fig. 1G–I View FIGURE ); papilla on FIV–FVI absent ( Figs 2A–C View FIGURE 2 ); presence of one papilla on FVII, FVIII and FIX ( Figs 2D–F View FIGURE 2 ); two papillae on FX ( Fig. 2G View FIGURE 2 ), presence of three, three, seven and six papillae distributed on FXI, FXII, FXIII and FXIV, respectively ( Figs 2H–K View FIGURE 2 ); preapical spiniform papilla observed on FIX ( Fig. 2F View FIGURE 2 ), FXI ( Fig. 2H View FIGURE 2 ), and FXIII ( Fig. 2J View FIGURE 2 ). Presence of simple setae on FIV– FXIV ( Figs 2A–K View FIGURE 2 ), Labial suture complete ( Fig. 1A View FIGURE ). Palpi (P) ( Fig. 1J View FIGURE ): palpal segment length: PI 35 (38/41, n = 2), PII 61 (55/61, n = 2), PIII 128 (116/125, n = 2), PIV 75 (70/72, n = 2) and PV 145 (125/148, n = 2) – Palpal formula: 1-2-4-3-5; PIII ( Fig. 1J View FIGURE ) with Newstead’s sensilla scattered along the segment .
Cervix: ventro-cervical sensillae absent. Cervical sclerite bearing a pair of spiniform sensilla.
Thorax: Mesonotum 599 (577, n = 1) in length. Mesonotum, pronotum, paratergite, anepisternum, metanotum and postnotum light brown, pleura off-white. Two proepimeral setae; 13 upper anepisternal setae. Setae present on the anterior margin of the katepisternum. Wing ( Fig. 2L View FIGURE 2 ): 2,294 ( 2,183 / 2,294, n = 2) in length, 664 (657/672, n = 2) in width; veins: R 5 1,350 ( 1.295 / 1,350, n = 3); alfa 518 (577/613, n = 2); beta 372 (298/311, n = 2); gamma 212 (136/200, n = 2); delta 96 (154/261, n = 2); pi 95 (66/125, n = 2). Legs (anterior; median; posterior): Coxae: 349 (339/363, n = 2), 353 (325/339, n = 2), 330 (316/335, n = 2); Femur: 876 (832/888, n = 2), 832 (814/832, n = 2), 943 (925, n = 1); Tibia: 1,369 ( 1,239 / 1,295, n = 2), 1,480 ( 1,332 / 1,350, n = 2), 1,628 (1,535, n = 1); Tarsomere I: 803 (740, n = 2), 869 (795/851, n = 2), 906 (851, n = 1). Sum of tarsomeres II+III+IV+V, 744 (715/730, n = 2), 759 (744/766, n = 2), 773 (788, n = 1).
Abdomen: 1,480 ( 1,424 / 1,776, n = 2) in length; tergal papillae absent. Spermathecae: 43 (41/43, n = 2) in length, 14 (14, n = 2) in width; with poorly defined rings ( Fig. 2M View FIGURE 2 ); Terminal knob: 12 (9/12, n = 2) in length and 12 (12, n = 2) in width, not individualized, with rounded shape and bristles in the apical region; common spermathecal duct: 70 (58/61, n = 3) in length and 6 (6, n = 2) in width, does not go beyond the middle of the genital fork; individual spermathecal duct: 162 (128/142, n = 2) in length and 6 (6, n = 2) in width, they are membranous with smooth walls and their widths are uniform throughout their length ( Fig. 2M View FIGURE 2 ). Cercus: 154 (154/159 n = 2) in length and 78 (69/78, n = 2) in width.
Material examined: 3 females (Nº 87550, 87016, 87549). Capture location: municipality of Lassance ( 17°53′22.31″ S, 44°34′53.63″ W), located in the state of Minas Gerais, Brazil. Collection date GoogleMaps : 2 females (87549, 87550) 08/ix/2008; 1 female (87106): 12/viii/2008. Trap: HP trap. Collector: Mariana Campos das Neves Farah Ramos. Complementary material: 2 females from “Parque Estadual do Rio Doce” ( 19º30′45″ S, 42º33′10″ W), state of Minas Gerais, Brazil. Collection date: from June 2003 GoogleMaps to July 2004. Trap: Malaise trap. Collector: Rogério Parentoni Martins. Material deposited at FIOCRUZ/COLFLEB.
Redescription of the Male. Head ( Fig. 3A View FIGURE 3 ) 335 (321/325, n = 2) in length, 270 (270/281, n = 2) in width. Arrangement of deciduous bristles in the occiput region forming an “X” shape; clypeus 122 (110/121, n = 2) in length; eyes 154 (151/154, n = 2) in length, 72 (72/84, n = 2) in width; interocular distance 125 (122/136, n = 2). Pharynx with streaks and no teeth ( Fig. 3B View FIGURE 3 ). Cibarium without teeth ( Fig. 3B View FIGURE 3 ). Labrum-epipharynx (LE) 136 (136/148, n = 2) ( Fig. 3C View FIGURE 3 ). Specimen with damaged (broken) antennas, description based on the other specimen (Nº 92113). Antenna ( Fig. 3D–F View FIGURE 3 , 4A–K View FIGURE 4 ): flagellomere length (F): FI 437 (404/469, n = 2), FII 227 (223/228, n = 2), FIII 223 (209/223, n = 2), FXIII 78 (81, n = 1) and FXIV 70 (67, n = 1). Ascoids not visible in specimen, description based on the other specimen (Nº 92113). Ascoids with short posterior spur and with a peduncle type of insertion, anterior spur is long in FI and reaches the level of the preapical papilla ( Fig. 3D View FIGURE 3 ), but does not reach FII; external ascoids implanted at the same level as the internal; presence of preapical papilla on FI–FIII ( Figs 3D–F View FIGURE 3 ); papillae on FIV–FVI absent ( Figs 4A–C View FIGURE 4 ); presence of one papilla on FVII and FVIII ( Figs 4D and E View FIGURE 4 ), two papillae on FIX ( Fig. 4F View FIGURE 4 ), three papillae on FX and FXI ( Figs 4G and H View FIGURE 4 ), presence of four, six, six papillae distributed on FXII, FXIII and FXIV, respectively ( Figs 4I–K View FIGURE 4 ); preapical spiniform papilla on FIX ( Fig. 4F View FIGURE 4 ), FXI ( Fig. 4H View FIGURE 4 ) and FXIII ( Fig. 4J View FIGURE 4 ). Presence of simple setae on FIV–FXIV ( Fig. 4A–K View FIGURE 4 ). Palpi damaged in specimen, description based on the other specimen (Nº 92113). Palpi (P) ( Fig. 3G View FIGURE 3 ): palpal segment length: PI 32 (29/38, n = 2), PII 52 (52, n = 2), PIII 107 (107, n = 2), PIV 55 (58/61, n = 2), PV 96 (96/116, n = 2). The length of PIII and PV varied between specimens, with the PIII being longer in some specimens, while others had the largest PV. Palpal formula: 1-2-4-(3-5) (n = 2); PIII with Newstead’s sensilla scattered along the segment ( Fig. 3G View FIGURE 3 ). Labial suture complete ( Fig. 3A View FIGURE 3 ).
Cervix. Ventro-cervical sensilla absent. Cervical sclerites bearing a pair of spiniform sensilla.
Thorax. Mesonotum 416 (416/423, n = 2) in length; pronotum, anepisternum, metanotum and postnotum light brown, paratergite and pleura off-white. Two proepimeral setae; seven-eight upper anepisternal setae. Setae on the anterior margin of the katepisternum present. Wing ( Fig. 4L View FIGURE 4 ): 1,776 ( 1,813 / 1,831, n = 2) in length, 467 (496/504, n = 2) in width; veins: R 5 1,504 ( 1,504 / 1,091, n = 2); alfa 344 (381/396, n = 2); beta 290 (234/278, n = 2); gamma 180 (194/206, n = 2); delta 17 (17/38, n = 2); pi 88 (80/131, n = 2). Legs (anterior, median, posterior): Coxae: 307 (298/307, n = 2), 288 (279/293, n = 2), 288 (292/297, n = 2); Femur: 752 (694/740, n = 2), 679 (684, n = 1), 760 (759, n = 1); Tibia: 1,147 ( 1,147 / 1,202, n = 2), 1,258 (1,313, n = 1), 1,369 (1,461, n = 1); Tarsomere I: 693 (701/777, n = 2), 760 (832, n = 1), 803 (851, n = 1). Sum of tarsomeres II+III+IV+V (anterior, median, posterior): 657 (642/693, n = 2), 679 (730, n = 1), 686 (737, n = 1).
Abdomen: 1,221 ( 1,350 / 1,535, n = 2) in length; tergal papillae absent. Terminalia ( Fig. 4M View FIGURE 4 ): gonocoxite 148 (145/148, n = 2) in length, 55 (55/58, n = 2) in width, without basal tuft of setae. Gonostyle 128 (130/142,1, n = 2) in length, without spiniform preapical seta and with four spines: one apical, one subapical (upper external), one lower external and one internal spine. The four spines are well-developed. Lower external spine implanted at a level closer to the upper external spine than the internal. Internal spine implanted in the apical third of the gonostylus. Paramere ( Fig. 4M View FIGURE 4 ): dorsal margin 128 (119/128, n = 2) and ventral 151 (148/151, n = 2) in length; basal level of implantation of the bristles of dorsal margin reaches the apical level implantation of the bristles in the ventral margin ( Fig. 4M View FIGURE 4 ). Parameral sheath sclerotized and coniform. Epandrial lobe 148 (146/148, n = 2) in length, 32 (29/32, n = 2) in width and rounded at the apex. Sperm pump ( Fig. 4N View FIGURE 4 ) 107 (107/119, n = 2); ejaculatory apodeme 78 (78/96, n = 2); aedeagal ducts with bevel shaped apex ( Fig. 4N View FIGURE 4 ), 397 (383/397, n = 2) in length and 3 (3, n = 2) in width; 3,7 times the length of the sperm pump. Cercus 119 (128/134, n = 2) in length, 49 (38/43, n = 2) in width.
Material examined: 3 males (Nº 89333, 89327, 89331). Capture location: municipality of Lassance ( 17°53′22.31″ S, 44°34′53.63″ W), located in the state of Minas Gerais, Brazil. Collection date: 26/iv/2010. Trap: CDC trap. Collector: not identified. Complementary material GoogleMaps : 2 males (Nº 92113, 92114) from “Parque Estadual do Rio Doce” ( 19º30′45″ S, 42º33′10″ W), state of Minas Gerais, Brazil. Collection date: from June 2003 to July 2004. Trap: Malaise trap. Collector: Rogério Parentoni Martins. Material deposited in the FIOCRUZ/COLFLEB GoogleMaps .
Distribution based on published articles: ARGENTINA: Resistencia, Chaco, Shannon & Del Ponte (1927); Misiones, Puerto Iguazú, Salomón et al. (2010: 69); Colonia Benítez; Margarita Belén, Szelag et al. (2017). COSTA RICA: Murilo & Zeledón (1985). COLOMBIA: Amazonas-Letícia; Boyacá-Puerto Boyacá; Caquetá-Solano; Tolima-Melga; Valle-Buenaventura, Lower Anchicaya Dam, Martins et al. (1978); La Macareña, Bejarano et al. (2006: 26). ECUADOR: Cañar-Conchacay; Guayas-Bucay, Cerro Cutuguay; Los Rios – Quevedo, La Montaña, Martins et al. (1978). FRENCH GUIANA: Approugue-Balourou, Guillaume, Saut Canori, Saut Machicou; Caux; Cayenne-Baduel, Cabossou, Crique, Anguille, Montabo, Rorota; Haute Mana-Souvenir; Iracoubo-Crique Blanche; Oyapock-Maripa, Saint Georges, Tampac, Martins et al. (1978). Montabo, Cayenne, French Guyana, Floch & Abonnenc (1943: 6). PANAMA: Darien-Altos de Quia, District de Pinogana; Panama canal area-Madden Forest Reserve, Martins et al. (1978). PERU: Cusco-Assunción, Pilcopata; Huánuco-Cachicoto; Loreto-Pucallpa, Serafin Filomeno, Zungarococha; Madre de Dios-Salvacíon, Alto Madre de Dios, Martins et al. (1978). REPUBLIC OF SURINAME: Sabajo Hills, Brokopondo, Kent et al. (2013: 6). TRINIDAD AND TOBAGO: Martins et al. (1978). VENEZUELA: Floresta Tropical úmida, Floresta Tropical Seca, Amazonas, Feliciangeli et al. (1988). BOLIVIA: Rio Yapacani, Santa Cruz, Bermudez & Young (1983: 5). BRAZIL: CEARÁ: Nova Olinda, Mangabeira (1942: 327). ACRE: Rio Branco, Martins et al. (1978). AMAPÁ: Macapá, Martins et al. (1978); Oiapoque, Santos et al. (2019: 14). AMAZONAS: Presidente Figueiredo, Alencar et al. (2011: 118); Terra Indígena Caititu, Lábrea, Silva et al. (2014). BAHIA: Parque Nacional do Pau-Brasil, Porto Seguro, Shimabukuro et al. (2016). ESPÍRITO SANTO: Vargem Alta, Pinto et al. (2009: 1). MARANHÃO: Cururupu; São Luís; Martins et al. (1978); Paço do Lumiar, Barros et al. (2000: 16); Barreirinhas, Rebêlo et al. (2010: 26); São Luís, Azevedo et al. (2011: 40); Águas do Miranda, Brilhante et al. (2015: 57); Santo Amaro, Pereira Filho et al. (2015): 8; Caxias, Guimarães & Silva et al. 2017. MATO GROSSO: Barra do Garças, Queiroz et al. (2012: 45); Sinop, Thies et al. (2018: 177). MATO GROSSO DO SUL: Corumbá, Galati et al. (1997: 31), Oliveira et al. (2016: 11), Barrios et al. (2019: 14); Campo Grande, Oliveira et al. (2003: 19), Galati et al. (2003: 47), Oliveira et al. (2006: 101), Dorval et al. (2016: 23); Bela Vista, Dorval et al. (2009; 2010). MINAS GERAIS: Timóteo, Andrade-Filho et al. (1997: 13); Pedra do Indaiá, Andrade-Filho et al. (2008: 37); Divinópolis, Margonari et al. (2010: 47), Nascimento et al. (2013: 125); Lassance, Carvalho et al. (2013; 2017); Terra Indígena Xacriabá, São João das Missões, Rêgo et al. (2015: 10), Costa et al. (2019: 52); Barra do Guaicuí, Várzea da Palma, Sanguinette et al. (2015: 8); Parque Estadual do Sumidouro, Saraiva et al. (2015: 10); Santuário do Caraça, Tonelli et al. (2017: 12); Ipatinga, Lana et al. (2018: 13); Rio Acima, Costa et al. (2021: 120). PARÁ: Belém, Martins et al. (1978); Bacarena, Oliveira et al. (2011: 106); Paraense; Santa Maria, Chagas et al. (2016: 7). PARANÁ: Itambaracá, Cruz et al. (2012: 45). PERNAMBUCO: Recife, Silva & Vasconcelos (2005: 38), Silva et al. (2020); São Vicente Férrer, Guimarães et al. (2012; 2014); Ipojuca, Miranda et al. (2015: 146); Iguaraçu, Silva & Vasconcelos (2005: 38), Silva et al. (2017: 26). PIAUÍ:Andrade-Filho et al. (2001: 96). RIO DE JANEIRO: Cantagalo, Peres Dias et al. (2016: 58). RIO GRANDE DO NORTE: Parnamirim, Pinheiro et al. (2013: 38); Nísia Floresta, Pinheiro et al. (2016: 9); Complexo Rio Doce, Silva et al. (2020). RONDÔNIA: Guajará Mirim; Porto Velho, Martins et al. (1978); Nova Mamoré, Parque Estadual Guajará-Mirim, Pereira-Júnior et al. (2019: 114); Ji-Paraná, Torchitte et al. (2020). SÃO PAULO: Botucatu, Cutolo et al. (2009: 18); Província Espeleológica do Vale do Ribeira, Parque Estadual Intervales, Galati et al. (2010: 54); Rio Claro, Cutolo et al. (2013: 19). TOCANTINS: Porto Nacional, Vilela et al. (2011: 6); Taquaraçu, Machado et al. (2012: 107).
Distribution based on the analyzed slides: BRAZIL. Amapá: Pedra Branca do Amapari; Ceará: Maranguape; Goiás: Itumbiara; Mato Grosso: Alta Floresta, Chapada dos Guimarães; Mato Grosso do Sul: Camapuã; Minas Gerais: Belo Horizonte, Divinópolis, Jaboticatubas, Jequitinhonha, Lassance, Pains, Paracatu, Monsenhor Paulo, São João das Missões, Santana do Riacho, Rio Acima, Tupaciguara, Várzea da Palma; Pará: Oriximiná; Pernambuco: Recife; Piauí: Curralinho, Miguel Leão; Tocantins: Porto Nacional.
Medical importance: Studies have not yet been conducted on this species regarding vector competence or capacity, or natural infection by parasites. Species feed in anurans ( Costa et al. 2021).
| LE |
Servico de Microbiologia e Imunologia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Phlebotominae |
|
Genus |
Sciopemyia sordellii ( Shannon & Del Ponte, 1927 )
| Chaves Júnior, Salvador P., Shimabukuro, Paloma H. F. & Andrade, Andrey J. 2022 |
Psychodopygus nordestinus
| Forattini, O. P. 1973: 475 |
Phlebotomus longicornutus
| Barretto, M. P. 1946: 534 |
Flebotomus nordestinus
| Young, D. G. & Morales, A. 1987: 662 |
| Mangabeira, O. F. 1942: 327 |
Phlebotomus sordellii
| Shannon, R. & Del Ponte, E. 1927: 730 |