Westwoodia ruficeps Brullé, 1846
|
publication ID |
https://doi.org/ 10.5281/zenodo.183505 |
|
DOI |
https://doi.org/10.5281/zenodo.5657776 |
|
persistent identifier |
https://treatment.plazi.org/id/F1678223-FFF3-FFE2-B6B3-A2387FEEFBFB |
|
treatment provided by |
Plazi |
|
scientific name |
Westwoodia ruficeps Brullé, 1846 |
| status |
|
Westwoodia ruficeps Brullé, 1846 View in CoL
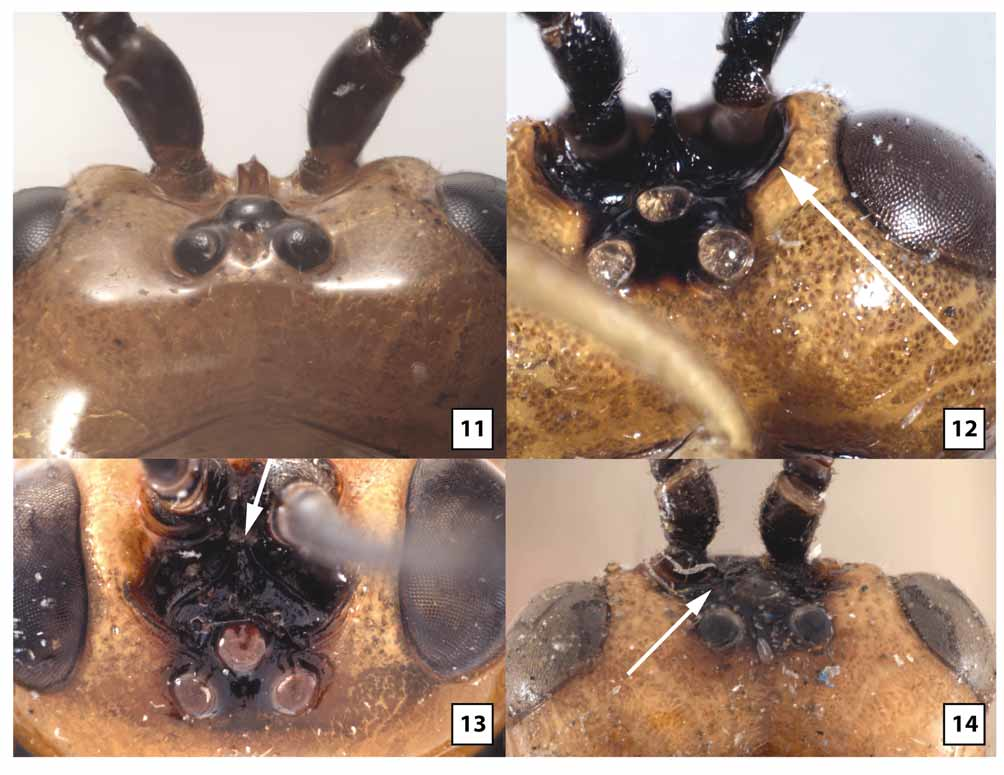
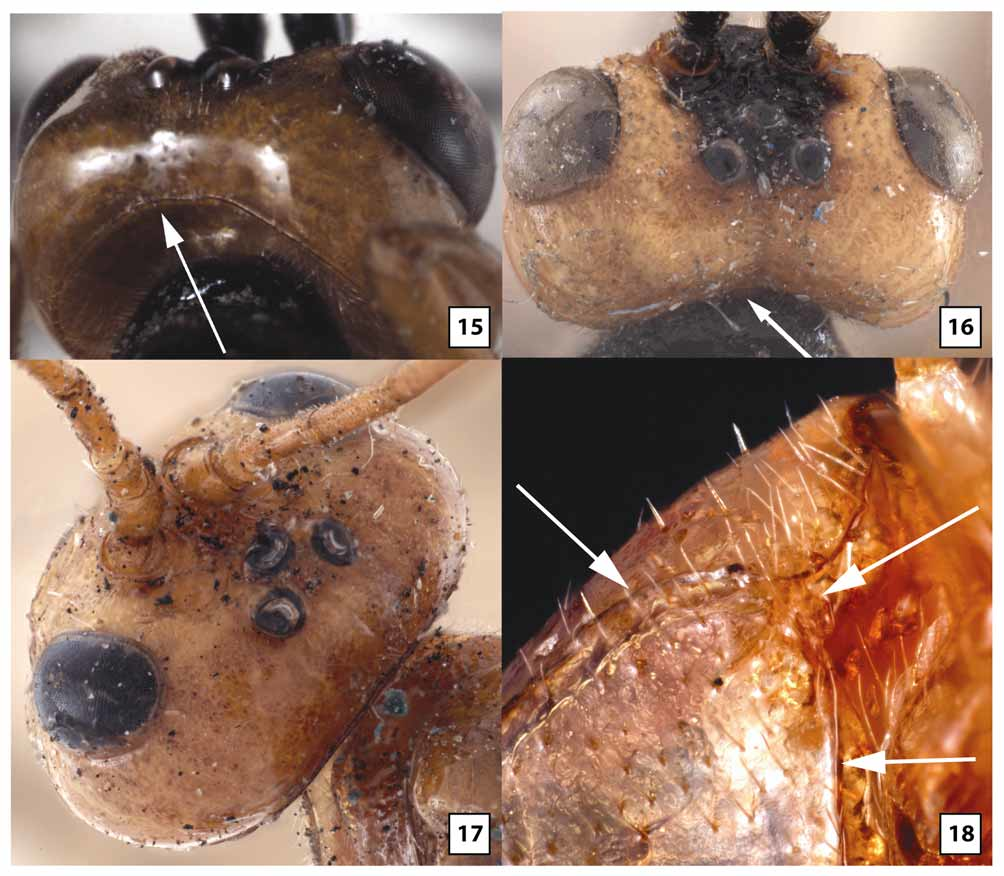
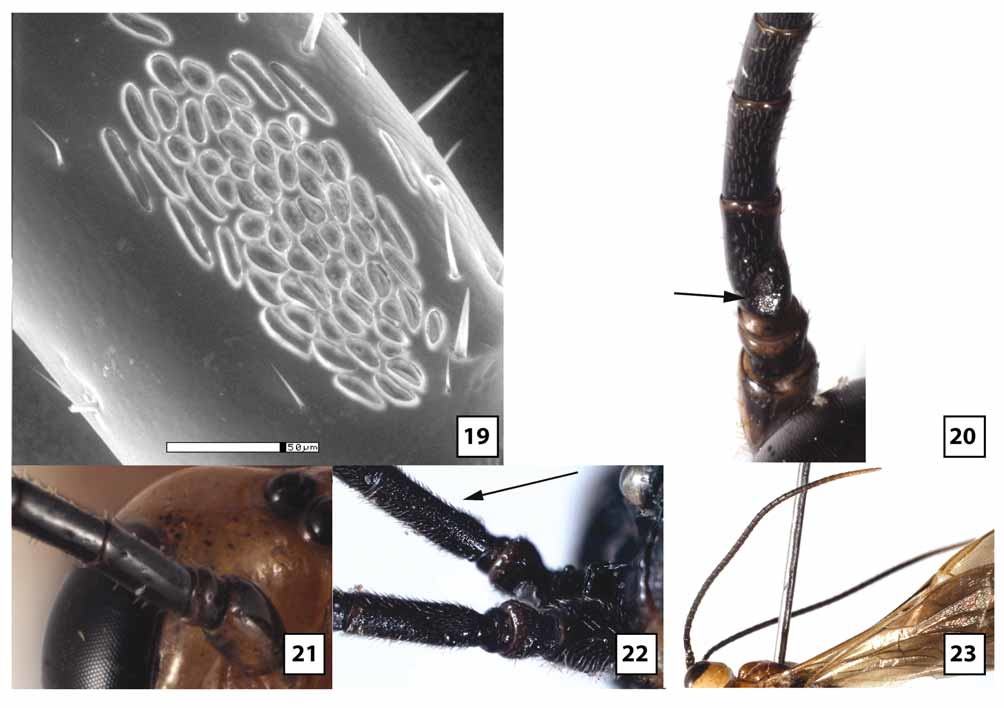
( Figs 5–6 View FIGURES 5 – 8 , 9 View FIGURES 9 – 10 , 11 View FIGURES 11 – 14 , 18–21, 23 View FIGURES 15 – 18 View FIGURES 19 – 23 , 27 View FIGURES 24 – 27 , 29 View FIGURES 28 – 31 , 33 View FIGURES 32 – 33 , 35 View FIGURES 34 – 37 , 38–39 View FIGURES 38 – 41 , 46–51 View FIGURES 46 – 49 View FIGURES 50 – 53 , 57–58 View FIGURES 54 – 57 View FIGURES 58 – 59 , 60–61, 63–66 View FIGURES 60 – 63 View FIGURES 64 – 66 )
Westwoodia ruficeps Brullé, 1846: 127 View in CoL –128 (original description); Dalla Torre 1901: 310 (catalog); Roman 1912: 240 – 241, 292–293 (identity); Morley 1913: 101 –102, 135–136 (redescription, figure, new distribution records); Viereck 1914: 152 (as type species of Westwoodia View in CoL ); Roman 1915: 4 –5 (diagnosis, key, first description of male, new distribution records); Townes et al. 1961: 213 (catalog, key to genera); Townes 1970: 58 (redescription of genus, figure, key to genera); Casolari and Casolari Moreno 1980: 59 (specimens in Spinola collection); Gauld 1984: 226 –227, 233– 234 (figures of species, redescription of genus, key to genera); Gupta 1987: 356 (catalog); Yu and Horstmann 1997: 456 (catalog).
Description
Female. Head ( Figs 5–6 View FIGURES 5 – 8 , 9 View FIGURES 9 – 10 , 11 View FIGURES 11 – 14 , 18–21, 23 View FIGURES 15 – 18 View FIGURES 19 – 23 , 66 View FIGURES 64 – 66 ): Face largely smooth, polished but usually with a few transverse wrinkles medially above epistomal sulcus (continuous with similar sculpture on clypeus), otherwise uniformly very sparsely punctate and setose. Clypeus usually transversely wrinkled at least dorsally and very sparsely punctate; ventral margin weakly emarginate medially, blunt to indistinctly carinate, abruptly but widely angled laterally; epistomal sulcus distinct. Eye in lateral view 1.0–1.2X longer than temple; malar space in frontal view 0.3–0.4X eye height, about equal to basal width of mandible; malar space sparsely punctate and setose, gena sparsely and weakly punctate. Frontal depression deep; lateral swelling of frons broad, well-developed, sparsely punctate, otherwise smooth; inner margin not carinate; frons medially with elongate, often hemispherical median flange extending to median ocellus. Occipital carina complete and well-developed throughout, curving abruptly towards mandible ventrally. Antenna with 32–37 flagellomeres, flagellum approximately 0.9–1.1X longer than fore wing; first flagellomere 2.2–2.8 (m = 2.5)X longer than wide, 1.0– 1.1X longer than second, setae very sparse, erect; second flagellomere 2.0–3.0X longer than wide; 10th flagellomere 1.2–1.8X longer than wide.
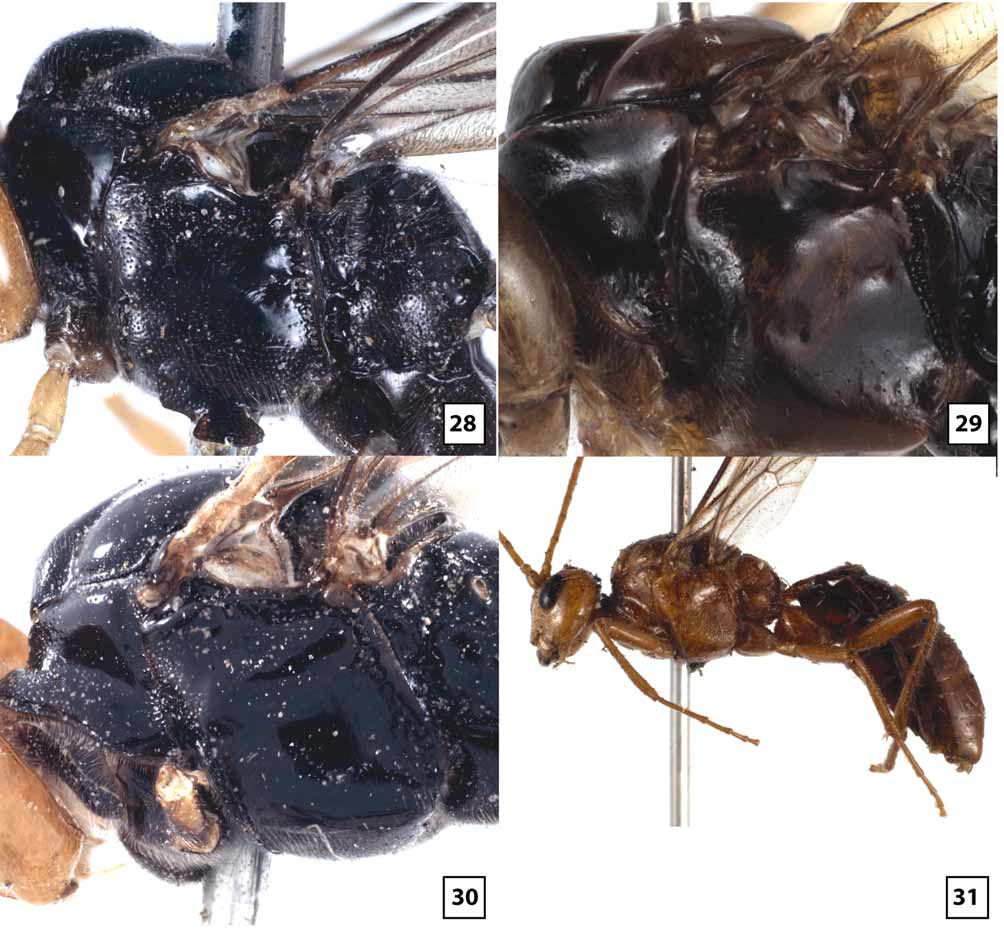
Mesosoma ( Figs 27 View FIGURES 24 – 27 , 29 View FIGURES 28 – 31 , 33 View FIGURES 32 – 33 , 35 View FIGURES 34 – 37 , 38–39 View FIGURES 38 – 41 , 46–49 View FIGURES 46 – 49 , 57–58 View FIGURES 54 – 57 View FIGURES 58 – 59 ): Dorsolateral margin of pronotum sparsely and weakly punctate, mostly smooth and polished. Notauli broad, deep at anterior margin, extending posteriorly onto dorsal surface of mesoscutal disc, shallow posteriorly to evanescent at junction with very shallow median depression; median lobe of mesoscutum distinctly elevated above lateral lobes anteriorly, with weak median longitudinal impression. Mesopleuron largely smooth and polished, very weakly and sparsely punctate and sparsely to moderately setose anteriorly and ventrolaterally. Propodeum glabrous or nearly so medially; pleural carina distinct, well-developed anteriorly but often poorly developed posteriorly; longitudinal carinae well-developed apically, almost as long as in W. gauldi , absent anteriorly. Fore wing areolet distinctly petiolate, 2m-cu arising well beyond middle, from proximal 0.65–0.80; 1cu-a postfurcal; CU1a inclivous, usually forming straight line with 2cu-a but sometimes forming weak angle. Fore basitarsus 1.2–1.7X longer than mid-width, 0.7–1.2X length of fifth tarsomere; second tarsomere of fore leg 1.0–1.4 (m = 1.1)X longer than wide; hind basitarsus 2.8–3.7X longer than wide, 1.7–2.3X longer than fifth tarsomere; second tarsomere of hind leg 2.0–2.2X longer than wide; fore tibia glabrous or nearly so dorsally and on much of posterior face, moderately setose ventrally, sparsely covered with stout setae anteriorly; hind tibia densely covered with decumbent setae posteriorly, more sparsely covered with stout, more erect setae anteriorly; hind and especially fore tarsomeres 1–4 broad and strongly compressed; fore tarsomeres nearly glabrous, with a few long setae except for dense patch of short, stout setae ventrally on basitarsus distad antennal cleaner; inflatable pads occupying most of ventral surface of fore tarsomeres 2–4; hind basitarsus densely setose posteriorly and ventrally, sparsely setose anteriorly, second tarsomere slightly less densely setose anteriorly and posteriorly, third and fourth tarsomeres with a few scattered setae, nearly glabrous; with inflatable pads occupying increasingly large portion, proximally to distally, of ventral surface of hind tarsomeres 2–4.
Metasoma ( Figs 64–65 View FIGURES 64 – 66 ): Petiole with distinct groove or depression between spiracle and posterior remnant of dorsal carina.
Color ( Figs 27 View FIGURES 24 – 27 , 63 View FIGURES 60 – 63 ): Head orange; mandibular teeth, frons medially, and ocellar triangle black, antenna black fading to dark brown near apex, apical flagellomere sometimes light brown. Mesosoma black; wings heavily infumate, stigma dark brown; legs dark reddish brown to black. Metasomal tergites black with apical margins very pale yellow, almost white, especially over distal half of metasoma; sternites very pale yellow to white, with large black blotches laterally.
Male ( Figs 50–51 View FIGURES 50 – 53 ) as in female except: eye (Qld) 1.1–1.4X longer than temple. Antenna with 32–36 flagellomeres, flagellum 1.0–1.2X longer than fore wing; first flagellomere 2.6–3.2X longer than wide, second flagellomere 2.8–3.4X longer than wide. Fore basitarsus 2.8–4.0X longer than mid-width, 1.0–1.2X longer than fifth tarsomere; third and fourth tarsomeres of fore leg 1.8–2.0 and 1.1–1.4X longer than wide, respectively; hind basitarsus 5.8–6.7X longer than wide; fore and hind tarsi more slender and densely setose throughout, inflatable pads smaller. Inner hind tibial spur shorter than apical width of tibia.
Body length: approximately 9.5–16.0 mm; fore wing 8.5–13.5 mm.
Hosts: Two species of Pergidae : Pergagrapta spinolae and P. polita , the latter collected on the host plants Eucalyptus propinqua and Melaleuca quinquenervia . See additional details and unconfirmed records under remarks.
Variation: We follow Gauld (1984) in interpreting W. ruficeps rather broadly. Some of the variation in morphology is readily categorized by population, and details are provided here to facilitate future work on this complex. Unfortunately, few populations are well represented in the material at hand, resulting in an emphasis in the paragraph below on comparisons between material from southern Queensland (Qld) and the type locality in Tasmania (Tas). A few comments are also offered on material from Victoria (Vic) and Canberra (ACT). Unfortunately, no males from Tasmania were available for examination.
The transverse wrinkling on either side of the epistomal sulcus tends to be more extensive in material from Qld than Tas, and five specimens from Vic and ACT lack this sculptural feature. In material from Vic and ACT, the clypeus is often more uniformly truncate (rather than medially emarginate) and somewhat flatter. The eye is variable in size, being longer and narrower in some individuals. Quantitative measures are provided from Tas in the description above. Comparable measures for Qld females show non-overlapping ranges, with eye in lateral view 1.2–1.8X longer than temple, and malar space in frontal view 0.5–0.6X eye height. Specimens from Tas also have more setae on the lower gena near the occipital carina, whereas setae are virtually non-existent in this area in Qld. The median, elevated, bladelike flange on the frons tends to be longer and more evenly hemispherical in Qld than Tas. In material from Tas, the flange more abruptly transitions to a low carina posteriorly as it extends to the median ocellus. Ten specimens from Vic have a tall, more knoblike interantennal projection that does not extend posteriorly and is much more similar in structure to that of W. gauldi than to W. ruficeps . Setae on the first flagellomere are somewhat more decumbent and a little less sparse posteriorly than anteriorly in Tas relative to Qld, and proportions of the second and 10th flagellomeres, as given in the above description, are at the upper end of the range (2.7–3.0 Tas vs 2.0–2.8 Qld and 1.7–1.8 Tas vs. 1.2–1.7 Qld, respectively). Specimens from Tas have the mesopleuron distinctly more setose than those from Qld, especially in the depression below the subalar ridge. Specimens from intermediate areas are variable, with some more closely resembling Tas and most more closely approaching Qld. The pleural carina of the propodeum is generally better developed in Qld than Tas. The range of values given in the description for origin of 2m-cu off the areolet is for Tas only to facilitate comparison among populations. In Qld and the reared series from ACT, 2m-cu generally arises from the extreme apex of the areolet (0.80–0.95X distance from base), while in a series from Vic, 2m-cu arises from the proximal 0.60–0.65. In this same Vic series, 2cu-a consistently forms a weak angle with CU1a. The areolet is variably petiolate within populations, as best exemplified by the long reared series from Qld where the stalk is very short, barely present in some and at least as long as the areolet in others. All specimens examined, regardless of locality, have short, stout tarsomeres on the fore leg with extensive fleshy pads ventrally. The hind legs are more variable, however, being a little longer and more slender in material from Vic, with hind leg basitarsus 4.3X (females) and 7.8–8.6X (males) longer than wide. Thus, hind tarsi of males from Vic fall within the range for W. rodmani but are still shorter than legs of the other three species treated above. The setal pattern of the posterior face of the second tarsomere of the hind leg is distinctly denser in Tas and some specimens from Vic than in Qld and the reared series from ACT. The color description above pertains to specimens from Tasmania, which are darker than all mainland material examined with the exception of a few specimens from Vic. Specimens from Qld nearly always have white to yellow fore tarsus, pale apical flagellomeres ( Fig. 23 View FIGURES 19 – 23 ), yellow fore wing stigma ( Fig. 61 View FIGURES 60 – 63 ), wings more weakly infumate basally, and pale petiole ( Fig. 64 View FIGURES 64 – 66 ) and T2. In approximately half of the mainland material, the mesosoma is largely pale yellow to orange, with propodeum and metapleuron mostly to entirely black, rarely (1%) entirely pale. Leg color varies within populations to some extent. Even within material from Qld, specimens reared from pergids on Eucalyptus plants have a higher percentage of the lightest colored individuals than specimens reared from pergids on Melaleuca plants. The neotype female from Hobart and the two specimens from the Spinola collection match the color pattern of W. ruficeps in Brullé’s original description, including the bicolored flange protruding upwards between the antennae and posteriorly onto the frons.
Material examined. Neotype Ψ, here designated (ANIC): [ AUSTRALIA, TASMANIA,] C.E. Cole Hobart 4.11.16 /2; second label = Ex. Coll. Nat. Mus.; third label = WESTWOODIA DET. IN MUS COLL. Additional specimens: ACT 8 Ψ 3 ɗ (MVMA) Black Mt, 28.i–10.iii.1937, Bred from Perga [grapta] polita larvae; 1 ɗ (MVMA) Black Mt., 19.ii.1936, A.D. Butcher; 1 Ψ (CNCI) Black Mt., 15–21.ii.1999, G. Gibson; 1 ɗ (ANIC) Brindabella Ra, 3.iii.1984, J.A. Vranjic and D. Horan ex Acacia melanoxylon overhanging creek, in copulation; 3 Ψ (ANIC) Canberra, 15.iv.1958, 4.ii.1959, and 16.ii.1960, E.F. Riek; 1 Ψ (TAMU), Canberra, 4– 10.i.1999, R. Wharton; NEW SOUTH WALES 1 ɗ (BMNH) Casula, 11.ii.1958, M.I. Nikitin; 1 Ψ (ANIC) Wilson’s Valley, 36.12S, 148.43E, 28.ii.1974, P. Morrow, predator on Pseudoperga sp. larvae; QUEEN- SLAND 1 ɗ (BMNH); 11 Ψ 5 ɗ (QMBA) Brisbane, Deagon, 27º20’S 153º04’E, 8.x.1997, S. Schmidt, larvae on Melaleuca quinquenervia , ex Pergagrapta nr. polita ; 12 Ψ 3 ɗ (QMBA) Brisbane, Deagon, 27º40’S 152º51’E, 21.viii and 30.ix.1997, S. Schmidt, larvae on Eucalyptus propinqua , ex Pergagrapta polita ; 1 ɗ (MVMA) Cape York, French; 1 Ψ (QMBA) Chelmer, 7.5 km SSW Brisbane, 18.viii.1992, M. Purcell; 1 Ψ (AEIC) Mt. Nebo, viii; 1 ɗ (AEIC) Nord-Queensland, E. Heyne; 1 ɗ (BMNH) Stanthorpe, iv.1927, H. Jarvis; TASMANIA 3 Ψ (ANIC) Hobart, 15.x.1916, C.E. Cole; VICTORIA 1 Ψ (USNM) F.M. Baker; 2 Ψ (ANIC) Ballarat, 28.v.1957, M.F. Leask; 1 ɗ (BMNH) Ballarat, emerged 11.vii.1957, M.F. Leask, foodplant of Symphyta, Eucalyptus ; 1 Ψ (BMNH) Ballarat, Glen Park, 11.xi.1956, M.F. Leask, actually in contact, a stride, clinging tenaceously to probably Pergagrapta bicolor , on Eucalyptus dives , “blue peppermint”; 4 ɗ (BMNH) Clunes, emerged iv–vi.1957, 7, 14& 18.iii.1958, M.F. Leask, ex pergid/Symphyta on Eucalyptus ; 1 Ψ (BMNH) Clunes, 10.ii.1959, M.F. Leask; 1 Ψ (BMNH) Clunes, 25.v.1959, M.F. Leask, bred from larvae, adults Pergagrapta spinolae ; 1 Ψ (MVMA) Meredith, 12.ii.1959, A. N.; WESTERN AUSTRALIA 1 ɗ (WAMP) Darlington, vii.1969, G.M. Lowe; 1 Ψ (WAMP) Glen Forrest [sic], 25.viii.1976, S.M. Postmus, feeding at flowers of Hakea amplexicaulis ; NO/UNCERTAIN LOCALITY 2 Ψ (MRSN); 5 Ψ 5 ɗ 1 sex unknown (ANIC, BMNH, MVMA); 1 Ψ (ANIC) Bulls HD, E. Pauci; 1 Ψ (ANIC) E. pauciflora (PR), 6.iii.1973, parasitic on Pseudoperga larva. Though this species occurs from Queensland to Tasmania, we have seen only a few individuals from NSW, and relatively few localities are represented by the material at hand.
Diagnosis
First flagellomere of antenna sparsely setose; interantennal flange typically tall, thin, and elongate, extending to or nearly to median ocellus, hemispherical in profile; lateral swelling of frons lacking carinate inner margin; face finely and sparsely punctate; occipital carina complete, distinctly developed throughout; female with tarsomeres of fore leg short, broad, and flattened; fore wing stigma dark brown in topotypic material, yellow in material from Queensland and some other localities; fore wing areolet present, with 2m-cu usually arising distinctly distad midpoint; fore and hind tarsi dark brown to black in topotypic material, mesosoma and metasoma dorsally, except for apical margin of tergites, black in topotypic material.
Distinguished from other species of Westwoodia by the distinctive interantennal flange ( Fig. 11 View FIGURES 11 – 14 ) and short, broad, strongly flattened fore tarsus with large, fleshy pads covering most of the ventral surface of tarsomeres 2–4 ( Figs 46–47 View FIGURES 46 – 49 , 57 View FIGURES 54 – 57 ). Though variable, the dorsal carina of the petiole tends to be better developed than in other species, often extending to the level of the spiracle as a distinct ridge. The antenna also tends to be shorter, with fewer flagellomeres in this species.
Remarks
Three distinct populations are evident in the material at hand: one from Tasmania, one from Brisbane, and one from south-central Victoria. In specimens from the type locality (Tasmania), the body is consistently darker than in specimens from southern Queensland, the interantennal flange is less evenly hemispherical, and the mesopleuron is not as sparsely setose. Specimens from areas in between are variable in these features, with some intermediate states, but a clear north-south cline was not evident. The reared series from Canberra, for example, is generally more similar to material from Brisbane than material from Tasmania. Most specimens from Canberra have the color and setal pattern of material from Brisbane, and most are reared from the same host, but the shape of the interantennal flange is variable and often more like material from Tasmania. One of the specimens from Canberra, however, matches the 10 specimens from Clunes, Meredith, and Ballarat that are differentiated from all other specimens by the shape of the interantennal flange and to a lesser extent by the shape and sculpture of the clypeus. It is quite possible that the material from south-central Victoria represents a separate species, but a few intermediates are present in the limited material at hand. We are thus reluctant to treat material from south-central Victoria as a separate species without more extensive collections from southeastern Australia to determine patterns of host utilization and whether there is any additional evidence of intergradation in form and color in this critical region. If the material examined from Victoria represents a second species, then it is possible that reared material from Canberra and Brisbane represent yet a third species that is sympatric with and may be host specific to Pergagrapta polita . The true W. ruficeps may thus be confined to Tasmania, outside the presently known distribution of P. polita . Host records are needed for material from Tasmania.
Westwoodia ruficeps View in CoL was described from a single female specimen from Van Dieman’s Land (= Tasmania), “Collect. de M. Serville” ( Brullé 1846). Many of the Brullé types from this work are in the Museum National d’Histoire Naturelle, Paris (MNHN), but it is also possible that Brullé returned the specimen to Jean- Guillaume Audinet-Serville just before the Serville Collection was sold to Massimiliano Spinola in 1847. The Brullé Collection in MNHN does not contain any specimens of Westwoodia View in CoL , and Townes et al. (1961) stated that the type was lost. The Spinola Collection, however, contains two specimens of Westwoodia View in CoL and although there are no locality labels, they match in every respect material from the type locality. It is thus possible that one of these specimens is the holotype, but there is no way to be certain, especially since the Westwoodia View in CoL specimens in the Spinola Collection apparently were obtained from Deyrolle rather than directly from Audinet-Serville ( Casolari & Casolari Moreno 1980). Because populations from Tasmania are distinct in at least some respects from mainland populations, and since repeated attempts have failed to provide any evidence that the holotype still exists, we have designated a specimen from Hobart as neotype. We have not selected one of the specimens from the Spinola collection as neotype because they are in poor condition.
Roman (1915) recorded a female from Fremantle (just south of Perth) and noted that since it was previously known only from Tasmania, this new record suggests that W. ruficeps View in CoL is distributed across the whole of South Australia. We have not seen Roman’s specimen from Fremantle, but two specimens in WAMP were collected from the vicinity of Perth. Morley (1913) recorded specimens from Adelaide and near Melbourne as W. ruficeps View in CoL . Unfortunately, his redescription is likely based on two different species (see comment above under W. longipes View in CoL ). Thus, we have confirmed records only from southwestern Australia around Perth, southeastern Australia (Tasmania and Victoria), and along the east coast from New South Wales to northern Queensland.
All known hosts belong to the subfamily Perginae ( Hymenoptera : Pergidae ). The host data provided above are based largely on 31 specimens from Brisbane reared from P. polita collected on the host plants E. propinqua and M. quinquenervia and 11 specimens from Canberra also from P. polita but without host plant data. There is also one specimen from Clunes reared from P. s p i n o l a e. Gauld (1984) also lists a record for Pseudoperga sp., and the label on this specimen indicates Eucalyptus pauciflora as the host plant. Unfortunately, the label does not include locality data, and six species of Pergidae , including two in Pseudoperga Guérin-Méneville and two in Pergagrapta Benson , are known from this host plant ( Schmidt & Smith 2006). Since some species now in Pergagrapta were formerly included in Pseudoperga ( Schmidt & Smith 2006) , the host for this record cannot be further pinpointed. There is also a specimen from Clunes that was observed attacking “probably” Pergagrapta bicolor (Leach) on Eucalyptus dives Schauer. Eucalyptus dives is not a known host for P. b i c o l o r, but two species of Pseudoperga are known from this host plant ( Schmidt & Smith 2006).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Westwoodia ruficeps Brullé, 1846
| Wharton, Robert A., Roeder, Karl & Yoder, Matthew J. 2008 |
Westwoodia ruficeps Brullé, 1846 : 127
| Yu 1997: 456 |
| Gupta 1987: 356 |
| Gauld 1984: 226 |
| Casolari 1980: 59 |
| Townes 1970: 58 |
| Townes 1961: 213 |
| Roman 1915: 4 |
| Viereck 1914: 152 |
| Morley 1913: 101 |
| Roman 1912: 240 |
| Dalla 1901: 310 |
| Brulle 1846: 127 |