Dipsastraea faviaformis ( Veron, 2000 ) Huang & Arrigoni & Benzoni & Fukami & Knowlton & Smith & Stolarski & Chou & Budd, 2016
|
publication ID |
https://doi.org/ 10.1111/zoj.12391 |
|
persistent identifier |
https://treatment.plazi.org/id/F25987C4-3131-FF96-FCBB-FDBBE09D6959 |
|
treatment provided by |
Marcus |
|
scientific name |
Dipsastraea faviaformis ( Veron, 2000 ) |
| status |
|
6. Echinophyllia Klunzinger, 1879: 69 View in CoL .
7. Homophyllia Br uggemann €, 1877: 310.
8. Micromussa Veron, 2000 , vol. 3: 8.
9. Moseleya Quelch, 1884: 292 .
10. Oxypora Saville Kent, 1871: 283 .
11. Sclerophyllia Klunzinger, 1879: 4 .
Taxonomic remarks
Lobophylliidae View in CoL was established by Dai & Horng (2009: 59) for six of the 13 genera in Mussidae sensu Veron (2000) View in CoL and two of the five genera in Pectiniidae sensu Veron (2000) . Licuanan (2009: 135) followed this scheme for the corals of the north-western Philippines. These taxa constitute the molecular clades XVIII, XIX, and XX designated by Fukami et al. (2008) (for a list of all available lobophylliid nomina, valid and synonymized, see Appendix S5).
For Mussidae sensu Veron (2000 View in CoL ; see also Vaughan & Wells, 1943; Wells, 1956), Dai & Horng (2009) dealt only with the fauna in Taiwan (i.e. Lobophyllia de Blainville, 1830: 321 View in CoL , Acanthastrea Milne Edwards & Haime, 1848a View in CoL , vol. 27: 495, Australomussa Veron, 1985: 171 View in CoL , Cynarina Br View in CoL uggemann €, 1877: 305, Scolymia Haime, 1852: 279 View in CoL , and Symphyllia View in CoL Milne Edwards & Haime, 1848a, vol. 27: 491), so the remaining seven genera were not included in the new family. The Atlantic taxa, represented by four of these seven genera, Mussa Oken, 1815: 73 View in CoL , Isophyllia View in CoL Milne Edwards & Haime, 1851a, vol. 5: 87, Mussismilia Ortmann, 1890: 292 View in CoL , and Mycetophyllia View in CoL Milne Edwards & Haime, 1848a, vol. 27: 491, were placed in Mussidae View in CoL by Budd et al. (2012) owing to the deep divergence between the Atlantic (clade XXI sensu Fukami et al., 2008 ) and Indo-Pacific fauna ( Fukami et al., 2004b, 2008), and the status of Mussa View in CoL as type genus of Mussidae Ortmann, 1890: 315 View in CoL . Blastomussa Wells, 1968: 276 View in CoL , was placed in family incertae sedis ( Budd et al., 2012) because it is genetically distinct from lobophylliids and mussids, and most closely related to Physogyra View in CoL , Plerogyra View in CoL , and Nemenzophyllia View in CoL (clade XIV; Fukami et al., 2008; Benzoni et al., 2014). Also in family incertae sedis is Indophyllia Gerth, 1921: 405 View in CoL , now considered an extinct genus after Indophyllia macassarensis Best & Hoeksema, 1987: 394 View in CoL , was transferred into Cynarina View in CoL by Budd et al. (2012). Micromussa Veron, 2000 View in CoL , vol. 3: 8, the final Mussidae View in CoL genus ( sensu Veron, 2000 ), was placed in Lobophylliidae View in CoL by Budd et al. (2012).
Further actions influenced the final generic composition of Lobophylliidae View in CoL prior to the present study. Scolymia View in CoL , one of the six genera that initially defined the family ( Dai & Horng, 2009), was moved into Mussidae View in CoL because its type, Madrepora lacera Pallas, 1766: 298 (see Vaughan, 1901: 6), is an Atlantic species ( Budd et al., 2012). Its two Indo-Pacific members were redis- tributed into Homophyllia Br View in CoL uggemann €, 1877: 310, and Parascolymia Wells, 1964: 379 View in CoL . The two Pectiniidae genera ( sensu Veron, 2000 ) initially assigned to Lobophylliidae View in CoL by Dai & Horng (2009), Echinophyllia Klunzinger, 1879: 69 View in CoL , and Oxypora Saville Kent, 1871: 283 View in CoL , were joined by Echinomorpha Veron, 2000 View in CoL , vol. 2: 333 ( Budd et al., 2012). Moseleya Quelch, 1884: 292 View in CoL , formerly in Faviidae sensu Veron (2000) View in CoL was also placed in Lobophylliidae View in CoL ( Huang et al., 2011; Budd et al., 2012). Sclerophyllia Klunzinger, 1879: 4 View in CoL , was resurrected based on new molecular and morphological data collected for Sclerophyllia margariticola Klunzinger, 1879: 4 View in CoL , whose sister congener is Acanthastrea maxima Sheppard & Salm, 1988: 276 ( Arrigoni et al., 2015) View in CoL . Arrigoni et al. (2014b) found Australomussa View in CoL and Parascolymia View in CoL to be genetically indistinguishable, and therefore considered the former to be a junior synonym of the latter. Finally, based on a morpho-molecular approach Arrigoni et al. (2016a) formally revised Homophyllia View in CoL and Micromussa View in CoL with the inclusion of H. bowerbanki View in CoL ( Milne Edwards & Haime, 1857), Micromussa lordhowensis ( Veron & Pichon, 1982) View in CoL , and Micromussa multipunctata ( Hodgson, 1985) View in CoL , as well as the new species Micromussa indiana Benzoni & Arrigoni View in CoL , and Micromussa pacifica Benzoni & Arrigoni. View in CoL The authors also established Australophyllia Benzoni & Arrigoni View in CoL , to accommodate the highly divergent A. wilsoni View in CoL .
Drawing upon the morphological and molecular phylogenies inferred in this study ( Fig. 2 View Figure 2 ), as well as prior work carried out by Budd et al. (2012) and Arrigoni et al. (2012, 2014b,c, 2015, 2016a), we classify Lobophylliidae species into 11 genera. The major change over the most recent proposals by Arrigoni et al. (2014b, 2015) is the placement of all members of subclade I ( sensu Arrigoni et al., 2014c ) in Lobophyllia ; our results show neither genetic nor morphological separation amongst Lobophyllia , Parascolymia , and Symphyllia . Furthermore, they support the transfers of Ac. ishigakiensis Veron, 1990: 132 , into Lobophyllia , and L. pachysepta Chevalier, 1975: 269 , into Acanthastrea , which we carry out here. Lobophyllia thus becomes the most species-rich genus in Lobophylliidae but with relatively limited genetic differentiation amongst species (see Arrigoni et al., 2014b: fig. 9, 2014c: fig. 1).
Lobophylliidae is widely distributed on reefs of the Indo-Pacific, and absent in the eastern Pacific.
Morphological remarks
There are five synapomorphies defining Lobophylliidae (bootstrap support of 95 and decay index of 5): (1) coenosteum spinose (likelihood of 1 based on the Mk1 model); (2) columellae discontinuous amongst adjacent corallites with lamellar linkage (likelihood 1.00); (3) tooth base at midcalice elliptical-parallel (likelihood 1.00); (4) tooth tip orientation parallel or forming multiaxial bulb (likelihood 1.00); and (5) thickening deposits in concentric rings with extensive stereome (likelihood 1.00). These comprise two macromorphological, two micromorphological, and one microstructural features. All of these characters strongly support the monophyly of Lobophylliidae and are monomorphic within the clade. Furthermore, the subcorallite characters unequivocally distinguish Lobophylliidae from Merulinidae , which has circular tooth base at midcalice, tooth tip orientated perpendicular to the septum or as multiaxial threads, and thickening deposits that are thick fibrous.
Mussidae (clade XXI) is an exclusively Atlantic clade, and in contrast to Lobophylliidae , has costate coenosteum, regular (pointed) midcalice tooth tip, transverse septal crosses (as clusters or carinae), and no extensive stereome thickening ( Budd et al., 2012).
GENUS LOBOPHYLLIA DE BLAINVILLE, 1830: 321 View in CoL
( FIG. 4)
Synonyms
Australomussa Veron, 1985: 171 View in CoL (type species: Australomussa rowleyensis Veron, 1985: 171 View in CoL , figs 23 – 25; original designation, Veron, 1985: 171); Palauphyllia Yabe, Sugiyama & Eguchi, 1936: 44 View in CoL (type species: Lobophyllia hataii Yabe et al., 1936: 44 View in CoL , pl. 26: fig. 3, pl. 28: figs 6, 7; original designation, Yabe et al., 1936: 44); Parascolymia Wells, 1964: 379 View in CoL (type species: Scolymia vitiensis Br uggemann €, 1877: 304; original designation, Wells, 1964: 379); Symphyllia View in CoL Milne Edwards & Haime, 1848a, vol. 27: 491 (type species: Meandrina sinuosa Quoy & Gaimard, 1833: 227 , pl. 18: figs 4, 5 = Mussa nobilis Dana, 1846: 187 , pl. 8: fig. 10 = Mussa recta Dana, 1846: 186 , pl. 8, figs 11, 11a; Matthai, 1928: 229; original designation, Milne Edwards & Haime, 1848a, vol. 27: 491).
Type species
Madrepora corymbosa Forskal, 1775: 137 ; subsequent designation, Matthai, 1928: 210.
Original description
Animaux actiniformes, pourvus d’une grande quantite de tentacules cylindriques, plus ou moins longs, sortant de loges coniques, a ouverture subcirculaire, quelquefois même alongees et sinueuses, partagees en un grand nombre de sillons par des lamelles tranchantes, laciniees, situees a l’extremite des branches, en general peu nombreuses et fasciculees, composant un polypier calcaire, fixe, turbine, strie longitudinalement a l’exterieur et tres-lacuneux a l’interieur. ( de Blainville, 1830: 321)
Subsequent descriptions
Quoy & Gaimard, 1833: 193; Milne Edwards & Haime, 1848a, vol. 27: 491; Milne Edwards & Haime, 1849a, vol. 11: 244; Milne Edwards & Haime, 1850, vol. 5: xxxii; Matthai, 1928: 208 – 210; Crossland, 1935: 502; Wells, 1936: 117; Yabe et al., 1936: 42 – 43; Vaughan & Wells, 1943: 194 – 195; Alloiteau, 1952: 630; Crossland, 1952: 142; Wells, 1956: F417; Nemenzo, 1959: 128; Chevalier, 1975: 231; Ditlev, 1980: 79; Veron & Pichon, 1980: 266; Scheer & Pillai, 1983: 145; Wood, 1983: 195 – 196; Veron, 1986: 412; Chevalier & Beauvais, 1987: 723 – 724; Veron & Hodgson, 1989: 267; Sheppard, 1990: 6; Sheppard & Sheppard, 1991: 116; Latypov & Dautova, 1998: 60 – 61; Veron, 2000, vol. 3: 38; Latypov, 2006: 343; Latypov 2014: 355.
Diagnosis (apomorphies in italics)
Colonial; submassive or massive. Budding intracalicular, and may also be extracalicular. Corallites monomorphic or polymorphic; discrete or uniserial. Monticules absent. Walls may be fused, or colonies may be phaceloid or flabello-meandroid. Calice width large (> 15 mm), with high relief (> 6 mm). Costosepta may or may not be confluent. Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes absent. Epitheca reduced if present. Endotheca abundant (vesicular) ( Fig. 4A, D, G, J, M).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Teeth tall (> 0.6 mm); widely spaced (> 1 mm), with> 6 teeth per septum. Tooth shape unequal between first- and third-order septa. Tooth size unequal between wall and septum. Granules scattered on septal face; weak (rounded). Interarea palisade ( Fig. 4B, E, H, K, N).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines weak. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 4C, F, I, L, O).
Species included
1. Lobophyllia corymbosa ( Forskal, 1775: 137) ; holotype: ZMUC ANT-000526 (dry specimen); type locality: Red Sea; phylogenetic data: molecular and morphology.
2. Lobophyllia agaricia ( Milne Edwards & Haime, 1849a, vol. 11: 255); holotype: MNHN scle913 (dry specimen); type locality: unknown; phylogenetic data: molecular and morphology.
3. Lobophyllia costata ( Dana, 1846: 179, pl. 7: figs 2, 2a, 2b); holotype: USNM 43 (dry specimen); type locality: Tahiti, Society Islands; phylogenetic data: molecular and morphology.
4. Lobophyllia dentata Veron, 2000 , vol. 3: 46, figs 1 – 4 (see also Veron, 2002: 134, figs 248, 249; ICZN, 2011: 164); lectotype (designated herein): MTQ G55826 (dry specimen); type locality: Milne Bay, Papua New Guinea (4 m depth); phylogenetic data: morphology only.
5. Lobophyllia diminuta Veron, 1985: 165 , figs 16, 17; holotype: WAM Z913 (also WAM 167 – 84; Griffith & Fromont, 1998: 236) (dry specimen); type locality: northern Swain Reefs, Australia (2 m depth); phylogenetic data: molecular and morphology.
6. Lobophyllia erythraea ( Klunzinger, 1879: 10, pl. 1: fig. 10, pl. 9: fig. 9); holotype: ZMB Cni 2171 (dry specimen); type locality: ‘ Kosseir’ (specimen label), Egypt, Red Sea; phylogenetic data: molecular and morphology .
7. Lobophyllia flabelliformis Veron, 2000 , vol. 3: 48, figs 1 – 5 (see also Veron, 2002: 136, figs 250 – 253; ICZN, 2011: 164); lectotype (designated herein): MTQ G55827 (dry specimen); type locality: Milne Bay, Papua New Guinea (7 m depth); phylogenetic data: molecular and morphology.
8. Lobophyllia grandis Latypov, 2006: 347 , fig. 80- 3 (= Lobophyllia sp. 1 : Latypov & Dautova, 1998: 64, pl. 14: fig. 3); holotype: FEBRAS 1/ 95279 (dry specimen); type locality: Bai Thanh Bay, Khanh Hoa, Vietnam (2.5 m depth); phylogenetic data: none.
9. Lobophyllia hassi ( Pillai & Scheer, 1976: 66, pl. 29: figs 2, 3); holotype: X2:88-6, Hessian State Museum , Darmstadt, status unknown; type locality: Rasdu Atoll, Maldives; phylogenetic data: none .
10. Lobophyllia hataii Yabe et al., 1936: 44 , pl. 26: fig. 3, pl. 28: figs 6, 7; holotype: TIU 56623 (dry specimen); type locality: Palau; phylogenetic data: morphology only .
11. Lobophyllia hemprichii ( Ehrenberg, 1834: 325) ; holotype: ZMB Cni 648 (dry specimen); type locality: Red Sea; phylogenetic data: molecular and morphology .
12. Lobophyllia ishigakiensis ( Veron, 1990: 132, figs 38 – 41, 80, 81); holotype: MTQ G32484 (dry specimen); type locality: Kabira Bay , Ishigaki Island, Japan (10 m depth); phylogenetic data: molecular and morphology .
13. Lobophyllia radians ( Milne Edwards & Haime, 1849a, vol. 11: 255); holotype: MNHN scle920 (dry specimen); type locality: ‘Ocean Indien’ (specimen label); phylogenetic data: molecular and morphology.
14. Lobophyllia recta ( Dana, 1846: 186, pl. 8, figs 11, 11a); syntype: USNM 9 (dry specimen); type locality: Wake Island, North Pacific Ocean ; phylogenetic data: molecular and morphology .
15. Lobophyllia robusta Yabe & Sugiyama in Yabe et al., 1936: 44, pl. 32: figs 2 – 4; holotype: TIU 40468 (dry specimen); type locality: Misaki , Shikoku, Japan; phylogenetic data: molecular and morphology .
16. Lobophyllia rowleyensis ( Veron, 1985: 171, figs 23 – 25); holotype: WAM Z907 (also WAM 171-84; Griffith & Fromont, 1998: 235) (dry specimen); paratypes: WAM Z908, Z909 (also WAM 172-84, 173-84; Griffith & Fromont, 1998: 235) (two dry specimens); type locality: Legendre Island, Dampier Archipelago, Western Australia (17 m depth); phylogenetic data: molecular and morphology.
17. Lobophyllia serrata Veron, 2000 , vol. 3: 41, figs 5, 6 (see also Veron, 2002: 133, figs 246, 247; ICZN, 2011: 164); lectotype (designated herein): UP MSI-3007-CO (dry specimen); type locality: Calamian Islands, Palawan, Philippines (10 m depth); phylogenetic data: none.
18. Lobophyllia valenciennesi ( Milne Edwards & Haime, 1849a, vol. 11: 256) (see Article 58.14 of the International Code of Zoological Nomenclature ); holotype: MNHN scle927 (dry specimen); type locality: Singapore; phylogenetic data: molecular and morphology .
19. Lobophyllia vitiensis (Br uggemann €, 1877: 304); holotype: NHMUK 1862.2.4.49 (dry specimen); type locality: Fiji; phylogenetic data: molecular and morphology.
Taxonomic remarks
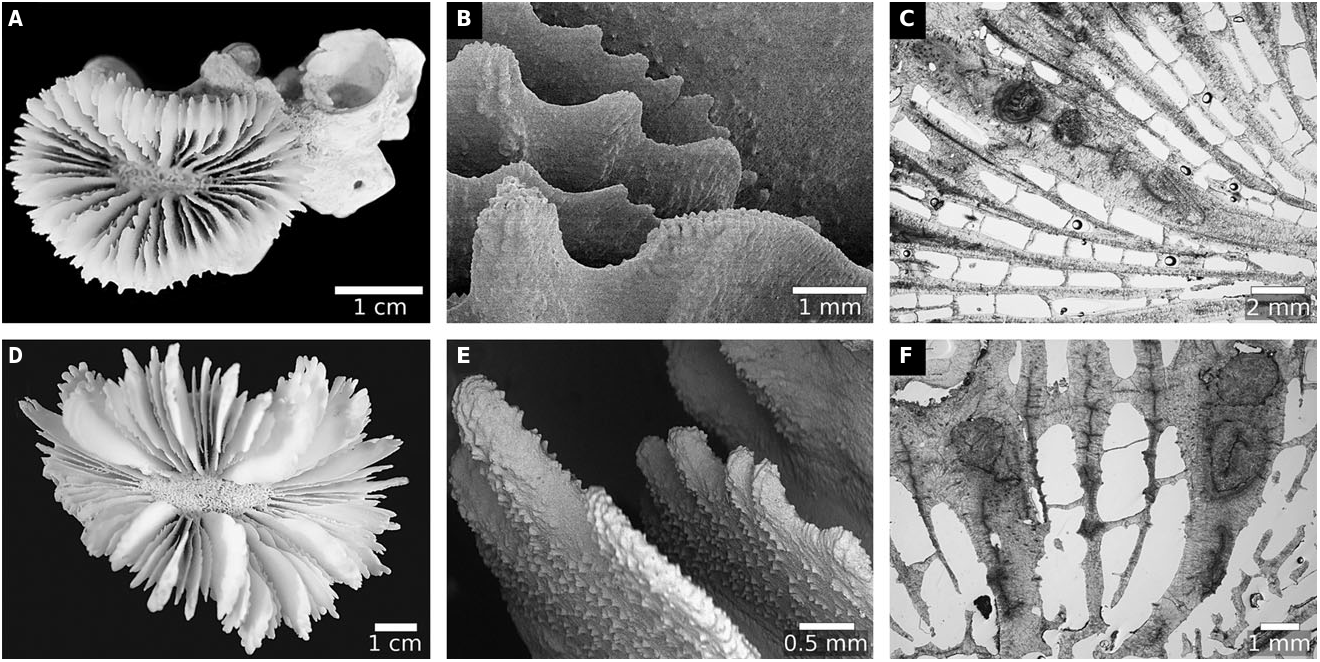
Lobophyllia View in CoL was first described by de Blainville (1830: 321) for seven species: (1) Lobophyllia glabrescens ( De Chamisso & Eysenhardt, 1821: 369) ; (2) Lobophyllia angulosa ( Pallas, 1766: 299) ; (3) Lobophyllia aurantiaca (= Lobophyllia aurea Quoy & Gaimard, 1833: 195 ); (4) Lobophyllia fastigiata ( Pallas, 1766: 301) ; (5) Lobophyllia corymbosa ( Forskal, 1775: 137) View in CoL ; (6) Lobophyllia sinuosa ( Lamarck, 1816: 229) View in CoL ; and (7) Lobophyllia carduus ( Ellis & Solander, 1786: 153) . The first, second, and fourth are the type species of Euphyllia Dana, 1846: 40 View in CoL , Mussa Oken, 1815: 73 View in CoL , and Eusmilia View in CoL Milne Edwards & Haime, 1848b, vol. 27: 467, respectively ( Matthai, 1928), whereas the third belongs to Tubastraea Lesson, 1829: 93 ( Cairns, 2001) View in CoL . The fifth species was thus chosen to be the type species of Lobophyllia View in CoL , and the genus resurrected by Matthai (1928: 208) to incorporate all the Indo-Pacific species of Mussa View in CoL as defined by Milne Edwards & Haime (1857), i.e. L. corymbosa ( Forskal, 1775: 137) View in CoL , L. costata View in CoL ( Dana, 1846: 179; but see Sheppard, 1987) and L. hemprichii ( Ehrenberg, 1834: 325) View in CoL . A further eight species were described in this genus by Yabe et al. (1936; two species), Chevalier (1975; one species), Veron (1985, 2000); four species), and Latypov (2006; one species).
However, our analyses demonstrate that L. pachysepta Chevalier, 1975: 269 View in CoL , is more closely related to Acanthastrea View in CoL than to other Lobophyllia species , including the type L. corymbosa View in CoL , and thus should be regarded as an Acanthastrea species ( Fig. 2 View Figure 2 ). Both molecular and morphological trees also show that Ac. ishigakiensis Veron, 1990: 132 View in CoL , Parascolymia View in CoL , and nearly all Symphyllia species are nested amongst Lobophyllia species in subclade I ( sensu Arrigoni et al., 2014c ), supporting the call by Arrigoni et al. (2014c) to consolidate these taxa into a single genus. Therefore, Ac. ishigakiensis View in CoL , both Parascolymia species , and six Symphyllia species are herein transferred into Lobophyllia View in CoL , which now comprises a clade of 19 closely related species. Many of these species form single lineages, but some are paraphyletic, including L. corymbosa View in CoL , L. hemprichii View in CoL , L. rowleyensis View in CoL , and L. vitiensis View in CoL (see Arrigoni et al., 2014b: fig. 9, 2014c: fig. 1).
The holotype of L. corymbosa , type species of Lobophyllia , is at the ZMUC (ANT-000526), where the types of other species described by Forskal (1775) can also be found today, e.g. lectotype of Dipsastraea favus ( Forskal, 1775: 132; ZMUC ANT-000466) and syntypes of Cyphastrea serailia ( Forskal, 1775: 135; ZMUC ANT-000367 to ANT-000373).
Lobophyllia View in CoL is widely distributed on the reefs of the Indo-Pacific, present from the Red Sea and East Africa to as far east as the Marshall Islands in the Northern Hemisphere ( Veron, 2000) and the Pitcairn Islands in the Southern Hemisphere ( Glynn et al., 2007).
Morphological remarks
This genus is delimited by two synapomorphies, uniserial corallites (likelihood of 1.00 based on the Mk1 model) and vesicular endotheca (likelihood 1.00). However, a reduction in the number of centres occurs amongst L. corymbosa , L. dentata , L. diminuta , and L. serrata . On the one hand, L. vitiensis and L. rowleyensis , previously in Parascolymia , form a clade that is supported by a moderate bootstrap value (71) and decay index (2), with the synapomorphies extracalicular budding (likelihood 1.00) and polymorphic corallites (likelihood 1.00). On the other hand, species that had in the past been separated into the genera Lobophyllia and Symphyllia ( sensu Matthai, 1928 ; Veron, 2000) do not form clades on either the morphological or molecular tree.
Symphyllia View in CoL has often been compared to Lobophyllia View in CoL , as both possess lamellar linkages between columellar centres ( Matthai, 1928; Vaughan & Wells, 1943; Wells, 1956), but the former can be differentiated by its longer, meandering valleys bordered by fused walls ( Chevalier, 1975; Wood, 1983; Veron, 1986, 2000). However, this distinction is problematic because Symphyllia valenciennesi Milne Edwards & Haime, 1849a, vol. 11: 256 (see Chevalier, 1975), and L. hataii Yabe et al., 1936: 44 View in CoL , have shallow and straight valleys that radiate from the colony centre, with the periphery being flabello-meandroid ( Veron, 2000). These two species do not group together on the morphological phylogeny ( Fig. 2B View Figure 2 ), but rather form a paraphyletic group with the rest of the Lobophyllia sensu stricto, indicating that these characters are not reliable in delimiting species groups within subclade I ( sensu Arrigoni et al., 2014c ).
Cynarina View in CoL is the sister genus of Lobophyllia View in CoL , but is morphologically distinct from the latter as it is solitary and may be free-living, have weak or moderate development of septal lobes, low-moderate (tabular) endotheca, and strong costa medial lines.
Although Lobophyllia View in CoL is restricted to the Indo- Pacific, it has historically been confused with the Atlantic genus Mussa View in CoL because they share many macromorphological characters ( Chevalier, 1975; Veron, 2000). However, the presence of lamellar linkages between columellar centres in Lobophyllia View in CoL , as mentioned above, is a key distinguishing feature ( Matthai, 1928). Furthermore, Mussa View in CoL possesses several subcorallite traits that are not found in Lobophyllia View in CoL : circular tooth base, pointed tooth tip, granules aligned on septal face, interarea formed by horizontal bands, parathecal walls with trabeculothecal elements, reduced thickening deposits, and transverse septal crosses ( Budd & Stolarski, 2009; Budd et al., 2012).
GENUS ACANTHASTREA MILNE EDWARDS & HAIME, 1848 View in CoL A: 495 ( FIG. 5 View Figure 5 )
Type species
Acanthastrea spinosa View in CoL Milne Edwards & Haime, 1848a, vol. 27: 495 = Astrea dipsacea Quoy & Gaimard, 1833: 210 , pl. 17: figs 1, 2 (see Dana, 1846: 226; Milne Edwards & Haime, 1849b, vol. 12: 145) (= Astraea echinata Dana, 1846: 229 , pl. 12: figs 1, 1a, b); original designation, Milne Edwards & Haime, 1848a, vol. 27: 495; holotype: MNHN IK-2010-599 (dry specimen); type locality: Tongatapu, Tonga.
Original description
Se separe de toutes les autres Astree s par ses cloisons tres-e chinulees dont les epines les plus fortes sont les plus exterieures. ( Milne Edwards & Haime, 1848a, vol. 27: 495)
Subsequent descriptions
Milne Edwards & Haime, 1849b, vol. 12: 144; Milne Edwards & Haime, 1850, vol. 5: xlii; Milne Edwards & Haime, 1851a, vol. 5: 106; Milne Edwards & Haime, 1857, vol. 2: 501; Klunzinger, 1879: 42; Duncan, 1884: 119 – 120; Delage & Herouard, 1901: 632; Vaughan, 1918: 125; Faustino, 1927: 162 – 163; Yabe et al., 1936: 47; Vaughan & Wells, 1943: 193 – 194; Alloiteau, 1952: 631; Crossland, 1952: 140 – 141; Wells, 1956: F417; Chevalier, 1975: 312; Ditlev, 1980: 79; Veron & Pichon, 1980: 252; Nemenzo & Hodgson, 1983: 42; Scheer & Pillai, 1983: 147; Wood, 1983: 195; Veron, 1986: 406; Chevalier & Beauvais, 1987: 724; Sheppard & Salm, 1988: 276; Veron & Hodgson, 1989: 266; Sheppard, 1990: 10; Sheppard & Sheppard, 1991: 112; Veron, 1993: 245; Latypov & Dautova, 1998: 59; Veron, 2000, vol. 3: 12; Claereboudt, 2006: 212; Latypov, 2006: 341; Latypov 2014: 353 – 354.
Diagnosis
Colonial; submassive or massive. Budding intracalicular and extracalicular. Corallites monomorphic; mainly discrete. Monticules absent. Coenosteum spinose; limited (includes double wall), moderate (<corallite diameter) amount, or colonies may be phaceloid or partly flabello-meandroid. Calice width medium to large (≥ 4 mm), with medium to high relief (≥ 3 mm). Costosepta mostly confluent. Septa in three cycles (24 – 36 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes usually absent. Epitheca reduced. Endotheca low-moderate (tabular) ( Fig. 5A, D, G View Figure 5 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Tooth height usually medium (0.3 – 0.6 mm). Tooth spacing medium to wide (≥ 0.3 mm), with ≤ 6 teeth per septum. Tooth shape unequal between first- and third-order septa. Tooth size equal between wall and septum. Granules scattered on septal face; weak (rounded). Interarea smooth ( Fig. 5B, E, H View Figure 5 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines weak. Septum centre clusters may be strong;> 0.5 mm between clusters; medial lines weak ( Fig. 5C, F, I View Figure 5 ).
Species included
1. Acanthastrea echinata ( Dana, 1846: 229, pl. 12: figs 1, 1a, b); syntype: USNM 25 (dry specimen); type locality: Fiji; phylogenetic data: molecular and morphology.
2. Acanthastrea brevis Milne Edwards & Haime, 1849b, vol. 12: 146; holotype: MNHN scle851 (dry specimen); type locality: unknown; phylogenetic data: none.
3. Acanthastrea hemprichii ( Ehrenberg, 1834: 320) ; holotype: lost; type locality: Red Sea; phylogenetic data: molecular and morphology .
4. Acanthastrea minuta Moll & Best, 1984: 53 , fig. 12; holotype: RMNH 15275 (dry specimen); type locality: 100 m offshore of north Bone Tambung , Spermonde Archipelago, Indonesia (7 m depth); phylogenetic data: none .
5. Acanthastrea pachysepta ( Chevalier, 1975: 269, pl. 24: fig. 1); holotype: MNHN IK-2010-660 (dry specimen); type locality: Chesterfield , Islands, New Caledonia (1 m depth); phylogenetic data: molecular and morphology .
6. Acanthastrea rotundoflora Chevalier, 1975: 325 , pl. 29: fig. 3, pl. 31: fig. 7; holotype: MNHN IK- 2010-675 (dry specimen); type locality: south-east Fabre Atoll , New Caledonia (4 – 5 m depth); phylogenetic data: molecular and morphology .
7. Acanthastrea subechinata Veron, 2000 , vol. 3: 13, figs 3 – 5 (see also Veron, 2002: 128, figs 238, 239; ICZN, 2011: 163); lectotype (designated herein): UP MSI-3001-CO (dry specimen); type locality: Calamian Islands, Palawan, Philippines (10 m depth); phylogenetic data: molecular only.
Taxonomic remarks
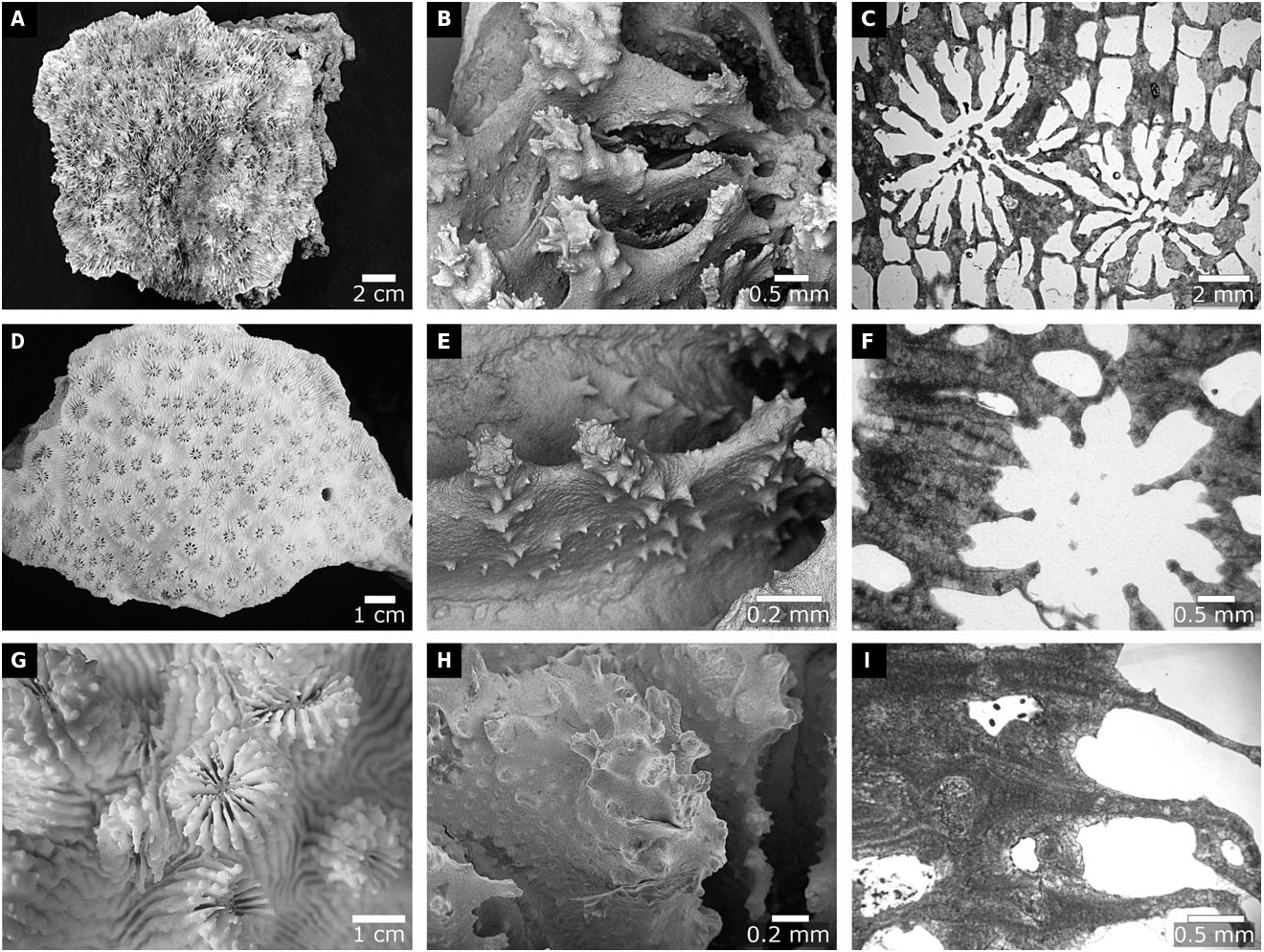
The genus was first described to contain four monocentric species (i.e. ‘ Astree s’; Milne Edwards & Haime, 1848a, vol. 27: 495) that have especially spinose wall septa – Acanthastrea hirsuta Milne Edwards & Haime, 1849b, vol. 12: 145, Acanthastrea spinosa Milne Edwards & Haime, 1848a, vol. 27: 495, Acanthastrea brevis Milne Edwards & Haime, 1849b, vol. 12: 146, and Acanthastrea grandis Milne Edwards & Haime, 1849b, vol. 12: 146. These species have mostly been synonymized as Acanthastrea echinata ( Dana, 1846: 229) ( Chevalier, 1975; Veron & Pichon, 1980). It should be noted that the Ac. spinosa specimen used by Milne Edwards & Haime, 1848a, vol 27: 495, to establish the genus (MNHN IK-2010-599) should still be considered the type of Acanthastrea .
By the time of Veron (2000), 12 Acanthastrea species were recognized as valid, including five described by Veron (1990, 2000) and Veron & Pichon (1982). Molecular phylogenetic analyses by Fukami et al. (2008) then showed that the genus was polyphyletic, with representatives in clades XVIII, clustering with Micromussa amakusensis ( Veron, 1990: 137) , and XX ( sensu Fukami et al., 2008 ). Kitahara et al. (2010) obtained a similar result, but extensive sampling by Arrigoni et al. (2014c) further showed that Acanthastrea is distributed amongst four major subclades (B, C, E, and I, sensu Arrigoni et al., 2014c ). Arrigoni et al. (2015) then swiftly moved Ac. maxima Sheppard & Salm, 1988: 276 , into the revived Sclerophyllia Klunzinger, 1879: 4 . Finally, Arrigoni et al. (2016a) synonymized Acanthastrea hillae Wells, 1955 , under Acanthastrea bowerbanki Milne Edwards & Haime, 1857, and moved the species into Homophyllia . Acanthastrea lordhowensis Veron & Pichon, 1982 , was also transferred into Micromussa , whereas Micromussa minuta ( Moll & Best, 1984) was moved into Acanthastrea based on detailed examination of the holotype ( Arrigoni et al., 2016a).
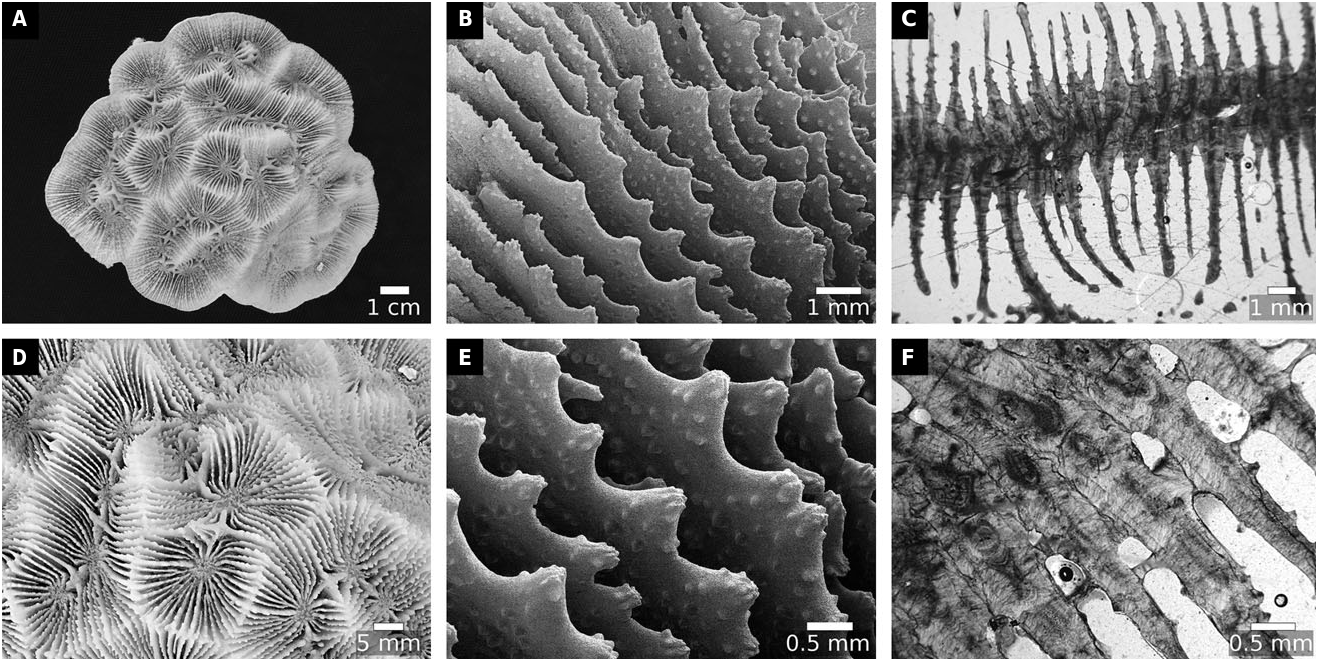
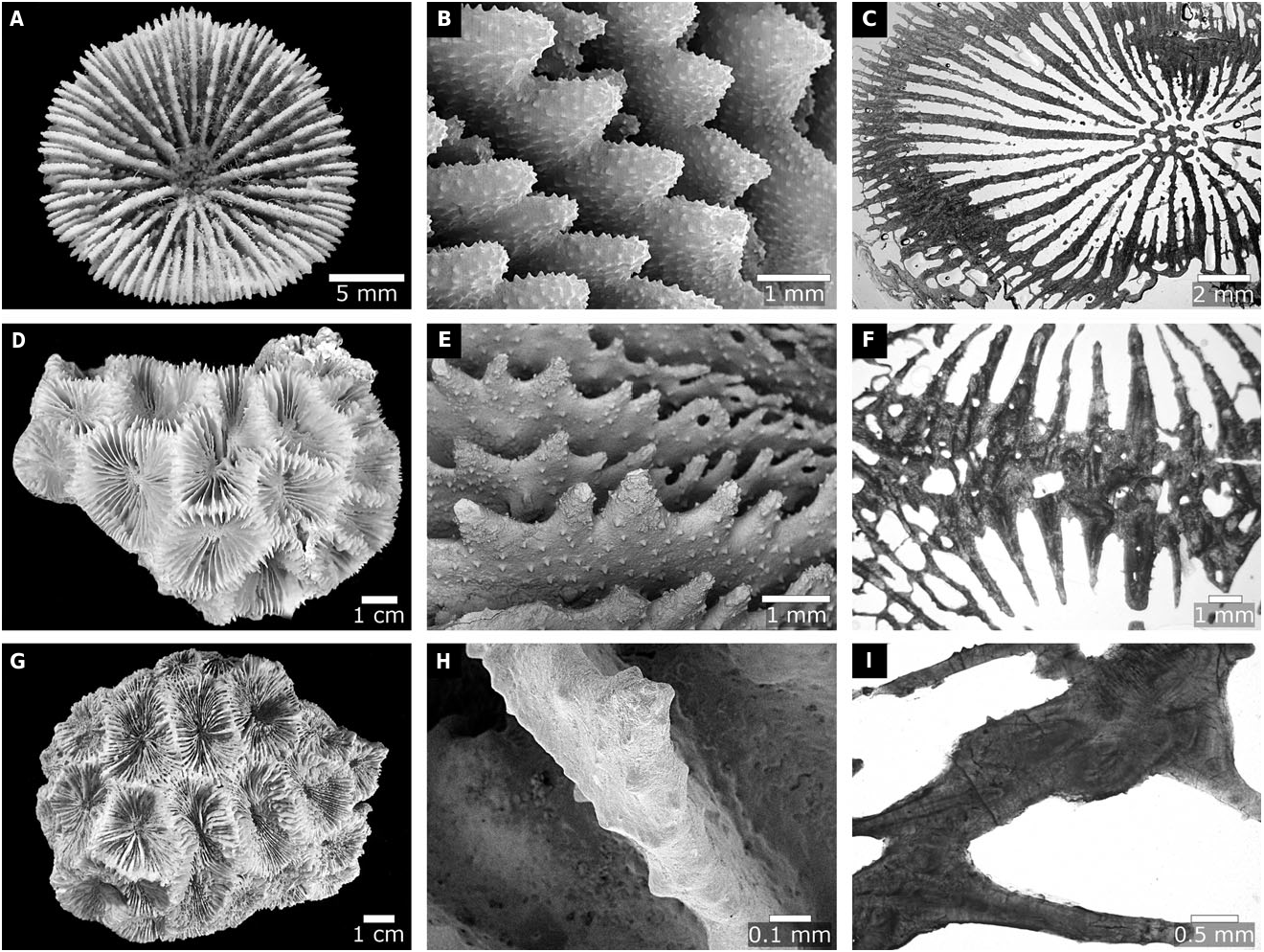
Our molecular and morphological trees support these changes, and also the further transfers of Ac. ishigakiensis Veron, 1990: 132 , into Lobophyllia ( Fig. 2 View Figure 2 ), and Ac. regularis Veron, 2000 , vol. 3: 16, into Micromussa . Arrigoni et al. (2014c) suggested that Ac. faviaformis Veron, 2000 , vol. 3: 24, should be transferred into the merulinid genus Dipsastraea de Blainville, 1830 , and our examination of the lectotype (designated herein) shows that its macromorphological characters are scored identically to Dipsastraea spp. (Appendix S2). Here we formally carry out the genus reassignment – Dipsastraea faviaformis ( Veron, 2000) comb. nov.
The molecular phylogeny here groups L. pachysepta Chevalier, 1975: 269 , and the remaining Acanthastrea species together in subclade E ( Fig. 2A View Figure 2 ), although they form a paraphyly on the morphological phylogeny ( Fig. 2B View Figure 2 ) owing to the disparately large corallites and phaceloid/flabello-meandroid colonies of L. pachysepta . Based on the molecular tree and subcorallite characters that are nearly identical between this rogue species and Acanthastrea – differing only in tooth spacing and distinctiveness of septum centre clusters – we move L. pachysepta into the present genus. The resulting classification thus comprises seven Acanthastrea species.
Acanthastrea View in CoL is widely distributed on the reefs of the Indo-Pacific, present from the Red Sea and East Africa to as far east as the Marshall Islands in the Northern Hemisphere ( Veron, 2000) and the Gambier Islands in the Southern Hemisphere ( Glynn et al., 2007).
Morphological remarks
The genus is paraphyletic on the morphological phylogeny ( Fig. 2B View Figure 2 ). On the molecular tree, Acanthastrea possesses several symplesiomorphies, including extracalicular budding, discrete corallites, columellae <1/4 of calice width, reduced epitheca, parallel tooth tip at midcalice, strong costa centre clusters, weak costa medial lines, and> 0.5 mm between septum centre clusters. These traits distinguish Acanthastrea from its sister clade of Echinophyllia + Oxypora . Excluding Ac. pachysepta , the genus is moderately supported on the morphological tree (bootstrap support of 68), with limited/moderate coenosteum amount and strong septum centre clusters as synapomorphies. Several characters separate Acanthastrea from taxa previously associated with the genus that are in subclades A ( Micromussa ), B ( Homophyllia ), C ( Sclerophyllia ), and I ( Lobophyllia ), including septa spacing, epitheca and endotheca development, number of teeth per septum, S1/S3 tooth shape, and wall/septum tooth size.
Acanthastrea View in CoL has historically been confused with the merulinid genus Favites Link, 1807: 162 View in CoL , as they are superficially alike and the inner edge of the septum possesses similar teeth ( Chevalier, 1975). When Matthai (1914) synonymized Favites View in CoL with Favia Oken, 1815: 67 View in CoL , the Acanthastrea species (i.e. Ac. hirsuta View in CoL and Astraea hemprichii ) were also transferred into Favia View in CoL , although these actions were almost immediately reversed as Vaughan (1918) revived both Favites View in CoL and Acanthastrea View in CoL . The latter is easily distinguished from Favites View in CoL by its sparser septa (three cycles; 24 – 36 septa; <6 septa per 5 mm), lamellar linkage between columellae, absence of paliform lobes, reduced epitheca and endotheca, less numerous septal teeth which are parallel to the septa at midcalice, smooth interarea, thickening deposits in concentric rings with extensive stereome, wider separation between centre clusters, and the lack of transverse crosses.
GENUS AUSTRALOPHYLLIA BENZONI & ARRIGONI IN ARRIGONI View in CoL ET AL., 2016A ( FIG. 6 View Figure 6 )
Type species
Symphyllia wilsoni Veron, 1985: 167 View in CoL , figs 18 – 22; original designation, Arrigoni et al., 2016a.
Diagnosis (apomorphies in italics)
Colonial; submassive or massive. Budding exclusively intracalicular. Corallites monomorphic; uniserial. Monticules may be present. Walls fused. Calice width usually medium (4 – 15 mm), with medium relief (3 – 6 mm). Costosepta mostly confluent. Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced six to 11 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes usually absent. Epitheca well developed. Endotheca low – moderate (tabular) ( Fig. 6A, D View Figure 6 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Tooth height medium (0.3 – 0.6 mm), but may be slightly taller. Tooth spacing medium (0.3 – 1.0 mm), with> 6 teeth per septum. Tooth shape equal between first- and third-order septa. Tooth size equal between wall and septum. Granules scattered, sometimes distributed uniformly, on septal face; weak (rounded). Interarea smooth ( Fig. 6B, E View Figure 6 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines weak. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 6C, F View Figure 6 ).
Species included
Australophyllia wilsoni View in CoL ( Veron, 1985: 167, figs 18 – 22); holotype: WAM Z910 (also WAM 168-84; Griffith & Fromont, 1998: 236) (dry specimen); paratypes: WAM Z911, Z912 (also WAM 169-84, 170-84; Griffith & Fromont, 1998: 236) (two dry specimens); type locality: Rat Island, Houtman Abrolhos Islands, Western Australia (8 m depth); phylogenetic data: molecular and morphology.
Taxonomic remarks
Australophyllia View in CoL was described by Benzoni & Arrigoni in Arrigoni et al. (2016a) to contain the phylogenetically distinct Symphyllia wilsoni Veron, 1985 View in CoL , as a newly discovered lineage (subclade J). Instead of grouping with its congenerics or the Lobophyllia species (subclade I) as defined in this study, it has been recovered close to Homophyllia View in CoL and Micromussa View in CoL based on molecular ( Arrigoni et al., 2016a; Fig. 2A View Figure 2 ) and morphological data ( Fig. 2B View Figure 2 ). No other species have been found with a closer relationship to Homophyllia View in CoL or Micromussa View in CoL despite near-complete sampling of the members of Symphyllia sensu Veron (2000) View in CoL .
Australophyllia View in CoL is restricted to the reefs of southern and Western Australia ( Veron, 2000; Arrigoni et al., 2016a).
Morphological remarks
Three autapomorphies, all macromorphological traits, unambiguously define this monotypic genus: exclusively intracalicular budding, presence of monticules, and uniserial corallites. Australophyllia is closely related to Homophyllia and Micromussa , forming a sister taxon to Homophyllia + Micromussa based on molecular data ( Fig. 2A View Figure 2 ), but a paraphyletic grade with morphological data, Micromussa being the earliest-branching clade ( Fig. 2B View Figure 2 ). As such, it appears to have an intermediate morphology between Micromussa and Homophyllia , particular with respect to calice width and relief, number of septa, and septal tooth height and spacing, as well as uniformity of granule distribution. It shares all other morphological traits (excluding the autapomorphies) with Homophyllia , therefore positioning it between Micromussa and Homophyllia in the grade.
Although it superficially resembles Symphyllia (= Lobophyllia ), in which Au. wilsoni was placed, it can be distinguished easily by the presence of monticules (or broken walls), smaller calices and septa spacing, well-developed epitheca, low – moderate endotheca, lower septal teeth and narrower tooth spacing, similar tooth shape between first- and third-order septa, and comparable tooth size between wall and septum, as well as smooth interarea.
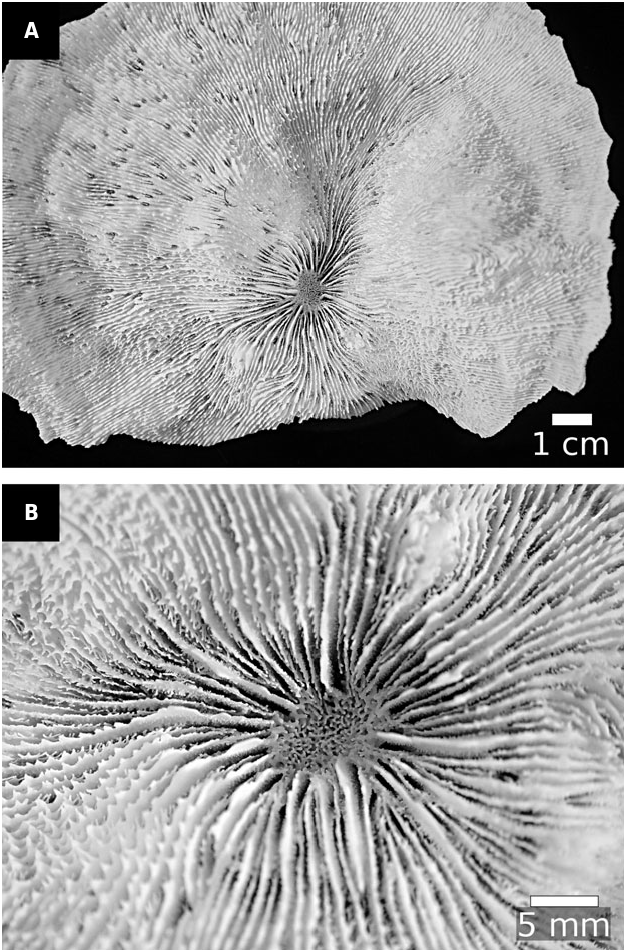
GENUS CYNARINA BR UGGEMANN €, 1877: 305 ( FIG. 7 View Figure 7 )
Synonyms
Acanthophyllia Wells, 1937: 242 (type species: Caryophyllia deshayesiana Michelin, 1850: 238 , pl. 2; original designation, Wells, 1937: 242); Protolobophyllia Yabe & Sugiyama, 1935: 381 View in CoL (type species: Antillia japonica Yabe & Sugiyama, 1931: 128 , pl. 37: figs 1 – 5, pl. 38: figs 1, 2; original designation, Yabe & Sugiyama, 1935: 382); Rhodocyathus Bourne, 1905: 191 View in CoL (type species: Rhodocyathus ceylonensis Bourne, 1905: 191 View in CoL , pl. 1: figs 1, 1A; original designation, Bourne, 1905: 191).
Type species
Cynarina savignyi Br View in CoL uggemann €, 1877: 305 = Caryophyllia carduus Audouin, 1826: 233 , pl. 4: figs 2.1, 2.2, 2.3 (= Caryophyllia lacrymalis Milne Edwards & Haime, 1849a, vol. 11: 238; Milne Edwards & Haime, 1848c, vol. 10, pl. 8: figs 1, 1a); original designation, Br uggemann €, 1877: 305; syntypes: NHMUK 1858.2.12.3, 1869.2.25.39, one unlabelled lot (eight dry specimens; Wells, 1964); type locality: Gulf of Suez, Red Sea.
Original description
Agreeing in all respects with Scolymia , except that the coral is free when adult, turbinate, and covered with a thick epitheca. From Antillia it differs in having the costae roughly spinose; the free edges of the larger septa lacerodentate, the septal teeth increasing in size from within outwards, the calicular fossa very shallow; the calice circular in the adult, compressed in the young (the reverse being the case in Antillia ). From Homophyllia it is likewise distinguished by the structure of its costae, septa, and fossa; besides, Homophyllia is always fixed by its base, and shows a very thin, appressed epitheca, whereas the latter is thick and only loosely adherent in Cynarina . (Br uggemann €, 1877: 305)
Subsequent descriptions
Klunzinger, 1879: 3 – 4; Wells, 1964: 376; Chevalier, 1975: 292; Ditlev, 1980: 76; Veron & Pichon, 1980: 238; Scheer & Pillai, 1983: 144 – 145; Wood, 1983: 193; Veron, 1986: 396; Chevalier & Beauvais, 1987: 723; Veron & Hodgson, 1989: 266; Sheppard, 1990: 6; Sheppard & Sheppard, 1991: 112; Veron, 1992: 148; Latypov & Dautova, 1998: 55 – 56; Veron, 2000, vol. 3: 82; Latypov, 2006: 338; Latypov, 2014: 350.
Diagnosis (apomorphies in italics)
Solitary. Budding intracalicular. Corallites monomorphic; discrete. Calice width large (> 15 mm), with high relief (> 6 mm). Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width. Septal (multiaxial) lobes weakly or moderately developed. Epitheca reduced. Endotheca usually low – moderate (tabular), but may be abundant ( Fig. 7A, D View Figure 7 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Teeth tall (> 0.6 mm); widely spaced (> 1 mm), with> 6 teeth per septum. Tooth shape unequal between first- and third-order septa. Tooth size unequal between wall and septum. Granules scattered on septal face; weak (rounded). Interarea palisade ( Fig. 7B, E View Figure 7 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines strong. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 7C, F View Figure 7 ).
Species included
1. Cynarina lacrymalis ( Milne Edwards & Haime, 1849a, vol. 11: 238; Milne Edwards & Haime, 1848c, vol. 10, pl. 8: figs 1, 1a); holotype: MNHN status unknown; type locality: ‘les Philippines?’ ( Milne Edwards & Haime, 1849a, vol. 11: 239); phylogenetic data: molecular and morphology.
2. Cynarina macassarensis ( Best & Hoeksema, 1987: 394, figs 5 – 7); holotype: RMNH 22189 (dry specimen); paratypes: RMNH 22190 – 22192 (seven dry specimens); type locality: Samalona , Spermonde Archipelago, Indonesia (21 – 36 m depth); phylogenetic data: morphology only .
Taxonomic remarks
Cynarina was established by Br uggemann € (1877: 305) for a new species Cynarina savignyi Br ugge- € mann, 1877: 305, which was collected from the Gulf of Suez and deposited at the British Museum (now NHMUK). Br uggemann € (1877: 306) stated on the description of Cyn. savignyi that, ‘of this species, the Museum contains a considerable series of specimens; yet I have taken the description from a single example, because this is the only one which is fully adult and at the same time beautifully regular in its septal apparatus’. Indeed , we found eight specimens at NHMUK that were examined by Br uggemann € (1877), and the largest of which fits his description and should be considered the holotype of the species ( Fig. 7A View Figure 7 ). However, Br uggemann € (1877: 305) was less specific in his description for the genus, and clearly used all of the specimens available to him at that time. Therefore we regard all eight specimens (NHMUK 1858.2.12.3, 1869.2.25.39, and one unlabelled lot) as syntypical material for the genus .
Cynarina savignyi View in CoL was named after J. C. Savigny, who discovered and figured the species as Caryophyllia carduus in Audouin (1826: 233, pl. 4: figs 2.1, 2.2, 2.3). The latter species name had already been used in Madrepora carduus Ellis & Solander, 1786: 153 , pl. 35 (= Madrepora lacera Pallas, 1766: 298 ), an Atlantic species, whereas Cyn. savignyi View in CoL was a junior synonym of Caryophyllia lacrymalis Milne Edwards & Haime, 1849a, vol. 11: 238, which remained the only valid species in Cynarina View in CoL until Budd et al. (2012) transferred Indophyllia macassarensis Best & Hoeksema, 1987: 394 View in CoL , into the genus. Our morphological analysis support this placement as Cyn. lacrymalis and Cynarina macassarensis View in CoL form a clade ( Fig. 2B View Figure 2 ), but molecular sampling is needed to verify this result.
Cynarina View in CoL has been affiliated with Lobophyllia View in CoL and Symphyllia View in CoL in the past. Matthai (1928) considered the solitary forms represented by Scolymia Haime, 1852: 279 View in CoL , Homophyllia Br View in CoL uggemann €, 1877: 310, Sclerophyllia Klunzinger, 1879: 4 View in CoL , and Cynarina View in CoL to be early monocentric stages of the colonial Lobophyllia View in CoL , and placed them in tentative synonymy under the latter. Wells (1937) followed this line of reasoning when he synonymized Scolymia View in CoL under Mussa Oken, 1815: 73 View in CoL , Homophyllia View in CoL under Lobophyllia de Blainville, 1830: 321 View in CoL , and Sclerophyllia View in CoL + Cynarina View in CoL under Symphyllia View in CoL Milne Edwards & Haime, 1848a, vol. 27: 491. Vaughan & Wells (1943) and Wells (1956) preserved this scheme but placed Cynarina View in CoL under Lobophyllia View in CoL instead. Subsequently, Wells (1964) resurrected all of the solitary taxa above except for Sclerophyllia View in CoL . The latter, together with Rhodocyathus Bourne, 1905: 191 View in CoL , and Protolobophyllia Yabe & Sugiyama, 1935: 381 View in CoL , were considered as synonyms of Cynarina View in CoL ( Wells, 1964; Veron & Pichon, 1980). However, the most recent phylogenetic analysis by Arrigoni et al. (2015), supported by our results here ( Fig. 2 View Figure 2 ), indicated that Sclerophyllia View in CoL is a distinct genus and it has since been resurrected (see below).
Acanthophyllia Wells, 1937: 242 , was described as a fully solitary coral that, in comparison with Cynarina View in CoL , possesses even larger lobate teeth, much bigger over the wall than near the columella. Although this separation was maintained by Wells (1964), Veron & Pichon (1980) studied the holotype of its type species Acanthophyllia deshayesiana and detected only minor differences in internal lobe development between Acanthophyllia and Cynarina View in CoL , tentatively listing Acanthophyllia as a junior synonym. Here, we also find septal tooth size and septal lobe development to be comparable between the two taxa, thus supporting the generic synonymy presented by Veron & Pichon (1980). Some exceptional specimens identified as Cyn. lacrymalis by Wells (1964, pls 20, 21) that were collected from Gubbins Reef in Australia and Banc Gail in New Caledonia have more rounded tooth tips and well-developed septal lobes. These peculiar corals have superficial affinities to Caryophylliidae View in CoL and are in need of more detailed examinations.
Cynarina View in CoL is widely distributed on the reefs of the Indo-Pacific, present from the Red Sea and East Africa to as far east as the Marshall Islands in the Northern Hemisphere and Samoa in the Southern Hemisphere ( Veron, 2000).
Morphological remarks
Two synapomorphies have been recovered for the moderately supported Cynarina clade (bootstrap support of 62): weakly or moderately developed septal (multiaxial) lobes (likelihood of 1.00 based on the Mk1 model) and strong costa medial lines (likelihood 1). The sister relationship between Cynarina and Lobophyllia recovered here is unsurprising given their previous affiliation, and the inclusive clade is indeed supported by the synapomorphy of unequal tooth size between the wall and septum (likelihood 0.90). They can however be distinguished easily based on Cynarina ’s synapomorphies, as well as its solitary form and low – moderate (tabular, instead of vesicular) endotheca.
Within Lobophylliidae View in CoL , in which species are predominantly colonial, Cynarina View in CoL is the only genus that is exclusively solitary. Lobophyllia vitiensis View in CoL (Br ugge- € mann, 1877: 304), Homophyllia australis View in CoL ( Milne Edwards & Haime, 1849a, vol. 11: 239), and Mi. pacifica Benzoni & Arrigoni View in CoL in Arrigoni et al., 2016a, are typically monostomatous but can sometimes form polystomatous coralla ( Arrigoni et al., 2014b; e.g. NHMUK 1840.11.30.79, syntype of Caryophyllia australis ). The congeneric of the monostomatous Sclerophyllia margariticola Klunzinger, 1879: 4 View in CoL – Scl. maxima ( Sheppard & Salm, 1988: 276) View in CoL – is colonial.
GENUS ECHINOMORPHA VERON, 2000 View in CoL (2): 333
( FIG. 8 View Figure 8 )
Type species
Echinophyllia nishihirai Veron, 1990: 130 View in CoL , figs 35 – 37, 79; original designation, Veron, 2000, vol. 2: 333.
Original description
This genus has only one species, see Echinomorpha nishihirai . ( Veron, 2000, vol. 2: 333)
For Echinomorpha nishihirai , ‘Characters: Colonies or individuals are thin and delicate. They may have only one corallite or have a prominent central corallite and widely spaced peripheral corallites. Septo-costae radiate from the central corallite like spokes from a wheel. Colour: Uniform or mottled dark browns or greens.’ ( Veron, 2000, vol. 2: 333)
Diagnosis (apomorphy in italics)
Colonial, but often solitary; laminar. Budding intracalicular. Corallites polymorphic; organically united and lacking distinct calical walls. Monticules absent. Coenosteum spinose; extensive amount (≥ corallite diameter). Calice width large (> 15 mm), with medium relief (3 – 6 mm). Costosepta mostly confluent in colonies. Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), ≥ 1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Paliform (uniaxial) lobes weakly developed. Epitheca absent. Endotheca low – moderate (tabular) ( Fig. 8 View Figure 8 ).
Species included
Echinomorpha nishihirai View in CoL ( Veron, 1990: 130, figs 35 – 37, 79); holotype: MTQ G32483 (dry specimen); type locality: Okinawa Island , Ryukyu Islands, Japan; phylogenetic data: morphology only .
Taxonomic remarks
Echinomorpha View in CoL is a monotypic genus that was described recently ( Veron, 2000, vol. 2: 333). Its sole member previously belonged to the closely related Echinophyllia View in CoL . Although no genetic material was available to place the genus on the molecular phylogeny, we analysed the macromorphological data for Echinomorpha nishihirai ( Veron, 1990: 130) View in CoL . Our results show that it is nested within the Echinophyllia View in CoL + Oxypora View in CoL clade and is the sister taxon to Echinophyllia tarae Benzoni, 2013: 63 View in CoL . There is low support for the latter relationship, but the former is supported by a high bootstrap value of 71 and decay index of 4. Owing to the sparse taxonomic sampling amongst Echinomorpha View in CoL , Echinophyllia View in CoL , and Oxypora View in CoL (subclade F + G sensu Arrigoni et al., 2014c ) in this study, we refrain from prescribing formal changes for these taxa.
Echinomorpha View in CoL is restricted to the reefs of the central Indo-Pacific between Japan and Indonesia ( Veron, 2000).
Morphological remarks
Echinomorpha possesses the autapomorphy of septa in ≥ 4 cycles (≥ 48 septa), and is unique amongst the closely related genera of Echinomorpha , Echinophyllia , and Oxypora in subclade F, which generally have fewer septa. Subcorallite and genetic characters for Echinomorpha nishihirai have not been examined, but all the observed macromorphological traits suggest that it may be the sister species of Echinophyllia tarae , which differs only in having a raised central corallite rim and paliform crown, and lacking the above autapomorphy ( Benzoni, 2013).
GENUS ECHINOPHYLLIA KLUNZINGER, 1879: 69 View in CoL
( FIG. 9 View Figure 9 )
Synonym
Oxyphyllia Yabe & Eguchi, 1935a: 377 View in CoL (type species: Madrepora aspera Ellis & Solander, 1786: 156 , pl. 39; original designation, Yabe & Eguchi, 1935a: 377).
Type species
Madrepora aspera Ellis & Solander, 1786: 156 , pl. 39; subsequent designation, Wells, 1936: 111.
Original description
Polypar zusammengesetzt, blattartig, d unn €, unten radi€ ar gerippt, oben mit zerstreuten mehr weniger vorstehenden Kelchen ohne deutliche Mauern, mit wohl entwickelten um die Kelchcentren radi aren € stark gez€ ahnten Septen; die Kelch durch stark gez ahnte € subparallele Rippen oder Septa verbunden. Columella deutlich, Unterseite gerippt, mit oder ohne Epithek. ( Klunzinger, 1879: 69)
Subsequent descriptions
Crossland, 1935: 503; Wells, 1936: 110 – 111; Vaughan & Wells, 1943: 197; Alloiteau, 1952: 631 – 632; Wells, 1955: 5; Wells, 1956: F419; Nemenzo, 1959: 119; Chevalier, 1975: 356 – 357; Pillai & Scheer, 1976: 67; Ditlev, 1980: 80; Veron & Pichon, 1980: 297 – 298; Scheer & Pillai, 1983: 152; Wood, 1983: 197 – 198; Veron, 1986: 372; Chevalier & Beauvais, 1987: 725 – 726; Sheppard, 1990: 16; Veron, 1993: 231; Latypov & Dautova, 1998: 43; Veron, 2000, vol. 2: 322; Claereboudt, 2006: 203; Latypov, 2006: 326; Latypov, 2014: 336.
Diagnosis
Colonial; laminar. Budding intracalicular; peripheral budding may be present. Corallites may be polymorphic; organically united and lacking distinct calical walls. Monticules absent. Coenosteum spinose; extensive amount (≥ corallite diameter). Calice width medium to large (≥ 4 mm), with low to medium relief (≤ 6 mm). Costosepta mostly confluent. Septa in ≤ 3 cycles (≤ 36 septa). Free septa irregular. Septa spaced ≤ 11 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), ≥ 1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Paliform (uniaxial) lobes weakly or moderately developed. Epitheca absent. Endotheca low – moderate (tabular) ( Fig. 9A, D, G View Figure 9 ).
Tooth base at midcalice elliptical-parallel. Tooth tip forming multiaxial bulb. Tooth height medium (0.3 – 0.6 mm). Tooth spacing medium (0.3 – 1.0 mm), with ≤ 6 teeth per septum. Tooth size equal between wall and septum. Granules scattered on septal face; weak (rounded). Interarea smooth ( Fig. 9B, E, H View Figure 9 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits with extensive stereome. Costa centre clusters weak;> 0.6 mm between clusters; medial lines strong. Septum centre clusters weak; 0.3 – 0.5 mm between clusters; medial lines weak ( Fig. 9C, F, I View Figure 9 ).
Species included
1. Echinophyllia aspera ( Ellis & Solander, 1786: 156, pl. 39); holotype: GLAHM 104004 (dry specimen); type locality: ‘Oceano Indiae orientalis’ ( Ellis & Solander, 1786: 156); phylogenetic data: molecular and morphology.
2. Echinophyllia costata Fenner & Veron in Veron, 2000, vol. 2: 330, figs 1 – 3 (see also Veron, 2002: 110, figs 209 – 212; ICZN, 2011: 163); lectotype (designated herein): MTQ G55809 (dry specimen); type locality: Banai Island, Sulawesi, Indonesia (22 m depth); phylogenetic data: morphology only.
3. Echinophyllia echinata ( Saville Kent, 1871: 283, pl. 23: fig. 3); holotype: NHMUK 1855.12.7.155 (dry specimen); type locality: San Cristobal, Solomon Islands; phylogenetic data: molecular and morphology .
4. Echinophyllia echinoporoides Veron & Pichon, 1980: 310 , figs 539 – 545, 806; holotype: NHMUK 1983.9.27.4 (dry specimen); type locality: Whitsunday Islands , Australia; phylogenetic data: molecular and morphology .
5. Echinophyllia orpheensis Veron & Pichon, 1980: 302 , figs 522 – 534, 803, 804; holotype: MTQ G57510 (dry specimen); type locality: south Pioneer Bay , Orpheus Island, Palm Islands, Australia (10 m depth); phylogenetic data: molecular and morphology .
6. Echinophyllia patula ( Hodgson & Ross, 1981: 173, fig. 3); holotype: UP C-538 (dry specimen); type locality: Maribago , Mactan Island, Cebu, Philippines (35 m depth); phylogenetic data: none .
7. Echinophyllia pectinata Veron, 2000 , vol. 2: 331, fig. 4 (see also Veron, 2002: 112, figs 213 – 215; ICZN, 2011: 163); lectotype (designated herein): UP MSI-3004-CO (dry specimen); type locality: Calamian Islands, Palawan, Philippines (25 m depth); phylogenetic data: none.
8. Echinophyllia tarae Benzoni, 2013: 63 , figs 2 – 8, 9a, b, 10b, d; holotype: MNHN IK-2012-8000 (dry specimen); type locality: Taravai Island , Gambier Islands, French Polynesia (10 m depth); phylogenetic data: molecular and morphology .
Taxonomic remarks
The genus was established by Klunzinger (1879: 69) for the type species Madrepora aspera Ellis & Solan- der, 1786: 156, as well as Trachypora lacera Verrill, 1864: 53 , under the family ‘Fungidae’ ( Klunzinger, 1879: 59). It was thought to be closely related to Halomitra Dana, 1846 , Mycedium Milne Edwards & Haime, 1851b, vol. 15: 130, and Echinopora Lamarck, 1816: 252 , of which only the first genus is indeed in Fungiidae Dana, 1846: 283 . The latter two are nested within Merulinidae Verrill, 1865: 146 ( Budd et al., 2012; Huang et al., 2014b). Prior to this, Ma. aspera was actually grouped with Tra. lacera Verrill, 1864: 53 , in the genus Trachypora Verrill, 1864: 53 (= Oxypora Saville Kent, 1871: 283 ), which was an attempt to distinguish these species from Halomitra and Echinopora .
The association of Echinophyllia , or its junior synonym Oxyphyllia Yabe & Eguchi, 1935a: 377 , with the fungiids persisted when Wells (1935) grouped it with Oxypora , Tridacophyllia de Blainville, 1830: 327 (= Pectinia de Blainville, 1825: 201 ), Mycedium , and Physophyllia Duncan, 1884: 118 , in Tridacophylliidae Thiel, 1932: 96, which was originally placed in Fungida (see Yabe & Eguchi, 1935b). Furthermore, Oxyphyllia (= Echinophyllia ) was placed in Echinoporidae Verrill, 1901: 132 , together with Echinopora and Mycedium by Yabe et al. (1936). However, Wells (1935) stated that Physophyllia , and by familial association, Echinophyllia is not in Fungiidae , and furthermore that there are no true synapticulae – a major synapomorphy of Fungiidae – in any of these genera.
When Pectiniidae was established by Vaughan & Wells (1943: 196) within Faviida for the five Tridacophylliidae genera above, there was little doubt that Echinophyllia View in CoL was distinct from fungiids (but see Matthai, 1948), which were characterized by fenestrate septa. Since then, this classification had become convention (e.g. Wells, 1956; Nemenzo, 1959; Chevalier, 1975; Wood, 1983; Veron, 2000) until the challenge posed by molecular data first revealed by Fukami et al. (2004b). Through extensive genetic sampling of Echinophyllia View in CoL in recent years, consensus that Echinophyllia View in CoL and Oxypora View in CoL are sister genera (subclade F + G sensu Arrigoni et al., 2014c ) nested within the Lobophylliidae View in CoL clade (XIX sensu Fukami et al., 2008 ) is emerging. The remaining three living genera in Pectiniidae are nested within Merulinidae View in CoL (clade XVII sensu Fukami et al., 2008 ), and thus Pectiniidae has been synonymized ( Budd et al., 2012; see also Huang et al., 2011, 2014b; Arrigoni et al., 2012).
The placement of Echinophyllia View in CoL in Pectiniidae was long held and appeared stable, so the rare note that it resembled an outgroup was particularly prominent. Chevalier (1975) observed that the septal tooth ornamentation is strong and similar to those in ‘Mussidae’ (= Lobophylliidae View in CoL ), becoming more irregular distally. Our character analysis supports this observation, with Echinophyllia View in CoL displaying similar tooth base and tip outline as other lobophylliids, but with the apex enlarging into a multiaxial bulb by branching into multidirectional tips.
Echinophyllia View in CoL is widely distributed on the reefs of the Indo-Pacific, present from the Red Sea and East Africa to as far east as the Marshall Islands in the Northern Hemisphere ( Veron, 2000) and the Gambier Islands in the Southern Hemisphere ( Glynn et al., 2007; Benzoni, 2013).
Morphological remarks
There are no unambiguous apomorphies for Echinophyllia on either the molecular or morphological tree. Three Oxypora species are nested amongst five Echinophyllia species in subclade F + G ( sensu Arrigoni et al., 2014c ) on the molecular phylogeny ( Fig. 2A View Figure 2 ), and these genera are not reciprocally monophyletic on the morphological tree ( Fig. 2B View Figure 2 ). The clade comprising these three genera is well supported with a bootstrap value of 71 and decay index of 4, and is defined by four synapomorphies: (1) organically united corallites (likelihood of 0.86 based on the Mk1 model); (2) extensive coenosteum (≥ corallite diameter) (likelihood 0.75); (3) columellae ≥ 1/4 of calice width (likelihood 0.92); and (4) loss of epitheca (likelihood 0.84).
The sister relationship between Echinophyllia and Oxypora is further supported by the presence of alveoli, which are small pits on the exotheca forming at points of insertion of new septocostae ( Chevalier, 1975; Wood, 1983; Veron, 1986, 2000; Benzoni, 2013). In Oxypora , these pits may penetrate to the undersurface of the colony to form slit-like pores ( Vaughan & Wells, 1943; Wells, 1956; Veron & Pichon, 1980; Dai & Horng, 2009). This distinction appears to be merely superficial as they cannot be distinguished based on molecular data or subcorallite morphology. Furthermore, the current Echinophyllia – Oxypora dichotomy belies the peculiar affinities of some constituent species. On the one hand, Echinophyllia echinata ( Saville Kent, 1871: 283) and Echinophyllia tarae Benzoni, 2013: 63 , are morphologically similar to Echinomorpha nishihirai – initially placed in Echinophyllia ( Veron, 1990) – mainly because they all possess a prominent central (polymorphic) corallite ( Benzoni, 2013). On the other hand, this affinity is not supported by either molecular or morphological data. More comprehensive taxonomic and genetic sampling of subclade F + G, especially of Oxypora species , would be necessary to resolve these genera.
Mycedium View in CoL was thought to be a closely related species to Echinophyllia View in CoL , and Wells (1954) remarked that the former can only be distinguished by its more inclined orientation of calices on laminar colonies. Detailed examinations of subcorallite morphology by Huang et al. (2014b) and the present study suggest that multiple characters separate them, including tooth base outline, tooth tip orientation, and thickening deposits, as well as costa and septum centre clusters.
GENUS HOMOPHYLLIA BRUGGEMANN View in CoL €, 1877: 310
( FIG. 10 View Figure 10 )
Type species
Caryophyllia australis Milne Edwards & Haime, 1849a, vol. 11: 239; Milne Edwards & Haime, 1848c, vol. 10, pl. 8: fig. 2; original designation, Br ugge- € mann, 1877: 310.
Original description
Coral neatly turbinate, with a narrow, somewhat expanded base. Outside of wall covered almost to the edge with a thin closely adherent epitheca, through which the costae are distinctly perceptible. Costae crowded, perfectly equal, prominent, minutely denticulate. Calicle circular, deep. Edges of septa with crowded, narrow, subequal teeth. Columella very small, rounded in outline, coarsely trabecular. (Br uggemann €, 1877: 310)
Subsequent descriptions
Wells, 1956: F417; Wells, 1964: 378; Ditlev, 1980: 76; Chevalier & Beauvais, 1987: 723; Arrigoni et al., 2016a.
Diagnosis (apomorphies in italics)
Colonial, but may be solitary in H. australis ; colonies submassive or massive. Budding intracalicular, and may also be extracalicular. Corallites typically monomorphic; discrete. Monticules absent. Walls fused. Calice width large (> 15 mm), with high relief (> 6 mm). Costosepta mostly confluent. Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced six to 11 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes usually absent. Epitheca well developed. Endotheca low – moderate (tabular) ( Fig. 10A, D, G View Figure 10 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Teeth tall (> 0.6 mm); widely spaced (> 1 mm), with> 6 teeth per septum. Tooth shape equal between first- and third-order septa. Tooth size equal between wall and septum, but the teeth at midcalice may be larger than those at the columellar end of the septum. Granules distributed uniformly on septal face; weak (rounded). Interarea smooth ( Fig. 10B, E, H View Figure 10 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines weak. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 10C, F, I View Figure 10 ).
Species included
1. Homophyllia australis ( Milne Edwards & Haime, 1849a, vol. 11: 239; Milne Edwards & Haime, 1848c, vol. 10, pl. 8: fig. 2); syntypes: NHMUK 1840.11.30.77, 1840.11.30.79 (two dry specimens); type locality: Port Lincoln, South Australia; phylogenetic data: molecular and morphology.
2. Homophyllia bowerbanki ( Milne Edwards & Haime, 1857, vol. 2: 503, pl. D6: fig. 1); holotype: MNHN scle850 (dry specimen); type locality: Australia; phylogenetic data: molecular and morphology.
Taxonomic remarks
Homophyllia View in CoL was established by Br uggemann € (1877: 310) to contain Caryophyllia australis Milne Edwards & Haime, 1849a, vol. 11: 239, the type and only one of two species to have been assigned to the genus until Arrigoni et al. (2016a) transferred into it a species previously in Acanthastrea View in CoL . Heterocyathus incrustans ( Dennant, 1906: 161) View in CoL , a junior synonym of the facultatively zooxanthellate Heterocyathus sulcatus ( Verrill, 1866: 48) View in CoL , was provisionally placed in Homophyllia View in CoL when it was first described ( Cairns, 2009).
The validity of Homophyllia View in CoL had been undermined for a considerable part of its taxonomic history. Matthai (1928) and Wells (1937) thought that it was an early monocentric stage of Lobophyllia View in CoL and therefore synonymized Homophyllia View in CoL under the latter. Vaughan & Wells (1943) did not question this scheme but Wells (1956) recognized it as a genus distinct from Lobophyllia View in CoL . Based on the similarity between Ca. australis Milne Edwards & Haime, 1849a, vol. 11: 239, and Scolymia vitiensis Br uggemann €, 1877: 304, Veron & Pichon (1980) placed both of them in Scolymia Haime, 1852: 279 View in CoL . Homophyllia View in CoL and Parascolymia Wells, 1964: 379 View in CoL , respectively contained these species, and were thus synonymized under Scolymia View in CoL . The authors were also not convinced that these two species were distinct, emphasising that ‘ H. australis and Scolymia View in CoL (= Parascolymia View in CoL ) vitiensis may be the same species, the former being a cold water ecomorph or geographic subspecies of the latter’ ( Veron & Pichon, 1980: 244). Nevertheless, they have remained as valid species to date, and were considered as the only Indo-Pacific members of Scolymia View in CoL ( Wood, 1983; Veron, 1986, 2000), whose type species Ma. lacera Pallas, 1766: 298 View in CoL (see Vaughan, 1901: 6), is an Atlantic species.
The deep divergence between the Atlantic (clade XXI sensu Fukami et al., 2008 ) and Indo-Pacific corals ( Fukami et al., 2004b, 2008) revealed by genetic data meant that the two Indo-Pacific members of Scolymia had to be redistributed into Homophyllia and Parascolymia ( Budd et al., 2012) . A more recent molecular analysis indicated that Ac. bowerbanki Milne Edwards & Haime, 1857, vol. 2: 503, and Ac. hillae Wells, 1955: 15 , are indistinguishable and form a sister group to H. australis , so Ac. hillae became a junior synonym of H. bowerbanki ( Arrigoni et al., 2016a) . Our analyses lend support to this classification ( Fig. 2 View Figure 2 ).
Homophyllia View in CoL is present on the reefs of the western Indian Ocean ( Sheppard & Sheppard, 1991) and central Indo-Pacific, to as far east as the Marshall Islands in the Northern Hemisphere ( Veron, 2000) and the Austral Islands in the Southern Hemisphere ( Glynn et al., 2007).
Morphological remarks
The Homophyllia clade comprising two species is moderately supported on the morphological tree ( Fig. 2B View Figure 2 ) with a bootstrap value of 63, as well as the synapomorphies of tall teeth (> 0.6 mm) (likelihood of 0.99 based on the Mk1 model) and granules distributed uniformly on the septal face (likelihood 1.00). It is the sister genus to Micromussa based on molecular characters ( Fig. 2A View Figure 2 ), but forms a paraphyletic group with Micromussa and Australophyllia on the basis of morphological traits ( Fig. 2B View Figure 2 ). Homophyllia is easily distinguished from these closely related genera by its larger and deeper calice, greater tooth height and spacing, and uniformly distributed granules.
Homophyllia australis View in CoL may be unique amongst congeneric and closely related allogeneric species in being predominantly solitary, but polystomatous specimens have been observed and collected ( Veron, 1986, 2000; Arrigoni et al., 2016a), including even one of its two syntypes, NHMUK 1840.11.30.79. In these cases, corallites may no longer be considered monomorphic as diagnosed for the genus. We also note that several coralla of H. bowerbanki View in CoL contain a central corallite that is slightly larger than usual.
GENUS MICROMUSSA VERON, 2000 View in CoL (3): 8 ( FIG. 11 View Figure 11 )
Type species
Acanthastrea amakusensis Veron, 1990: 137 View in CoL , figs 42 – 44, 82; original designation, Veron, 2000, vol. 3: 8.
Original description
Colonies are submassive or encrusting and usually flat. Corallites are cerioid or subplocoid, either circular or angular in shape and up to 8 millimetres diameter. Septa are thickened at the corallite wall, and have conspicuous teeth. Colonies may have fleshy tissue over the skeleton, but skeletal structures remain visible. Tentacles are extended only at night. ( Veron, 2000, vol. 3: 8)
Subsequent descriptions
Claereboudt, 2006: 226; Arrigoni et al., 2016a.
Diagnosis (apomorphies in italics)
Colonial; encrusting or massive. Budding intracalicular and extracalicular. Corallites monomorphic; discrete. Monticules absent. Coenosteum spinose; usually limited (includes double wall). Calice width medium (4 – 15 mm), with medium relief (3 – 6 mm). Costosepta mostly not confluent. Septa typically in three cycles (24 – 36 septa), although Mi. pacifica may contain more than 36 septa. Free septa irregular. Septa spaced six to 11 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes usually absent. Epitheca well developed. Endotheca low – moderate (tabular) ( Fig. 11A, D View Figure 11 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Tooth height medium (0.3 – 0.6 mm). Tooth spacing medium (0.3 – 1.0 mm), with> 6 teeth per septum. Tooth shape equal between first- and third-order septa. Tooth size equal between wall and septum. Granules scattered on septal face; strong (pointed). Interarea smooth ( Fig. 11B, E View Figure 11 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong; 0.3 – 0.6 mm between clusters; medial lines weak. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 11C, F View Figure 11 ).
Species included
1. Micromussa amakusensis ( Veron, 1990: 137, figs 42 – 44, 82); holotype: MTQ G32485 (dry specimen); type locality: Amakusa Islands , Japan (10 m depth); phylogenetic data: molecular and morphology .
2. Micromussa indiana Benzoni & Arrigoni in Arrigoni et al., 2016a; holotype: MNHN IK-2012- 14232 (dry specimen); type locality: Al Mukallah , Yemen (5 m depth); phylogenetic data: molecular and morphology .
3. Micromussa lordhowensis ( Veron & Pichon, 1982: 138 = Acanthastrea sp. Veron & Done, 1979: 219 = Acanthastrea sp. Veron & Pichon, 1980: 264, figs 455, 456); holotype: MTQ G57483 (dry specimen); type locality: North Bay, Lord Howe Island, Australia (2 m depth); phylogenetic data: molecular and morphology.
4. Micromussa multipunctata ( Hodgson, 1985: 284, figs 1 – 8, 9A); syntypes: UP C-783 , C-786 , C-787 , C-788 (four dry specimens); type locality: Tambuli Reef , Mactan Island, Cebu, Philippines (6 m depth); phylogenetic data: molecular and morphology .
5. Micromussa pacifica Benzoni & Arrigoni in Arrigoni et al., 2016a; holotype: MNHN IK- 2012-16043 (dry specimen); type locality: Mangareva , Gambier Islands, French Polynesia (15 m depth); phylogenetic data: molecular and morphology .
6. Micromussa regularis ( Veron, 2000, vol. 3: 16, figs 1 – 4; see also Veron, 2002: 130, figs 240 – 242; ICZN, 2011: 163); lectotype (designated herein): MTQ G55818 (dry specimen); type locality: Milne Bay, Papua New Guinea (3 m depth); phylogenetic data: none.
Taxonomic remarks
Micromussa View in CoL was established recently by Veron (2000, vol. 3: 8) to contain the designated type Acanthastrea amakusensis Veron, 1990: 137 View in CoL , as well as Ac. minuta Moll & Best, 1984: 53 View in CoL , and a new species Micromussa diminuta Veron, 2000 , vol. 3: 9. No data exist for the latter two species, but detailed observations by Arrigoni et al. (2016a) indicate that Ac. minuta View in CoL should not have been moved into Micromussa View in CoL , while Mi. diminuta actually belongs to Goniopora View in CoL . Molecular analyses have also demonstrated that Acanthastrea lordhowensis Veron & Pichon, 1982: 138 View in CoL , and Montastrea multipunctata Hodgson, 1985: 284 , are closely related to Mi. amakusensis View in CoL ( Arrigoni et al., 2014b,c, 2015, 2016a; see also Fig. 2A View Figure 2 ). Specifically, Montastrea multipunctata is closely related to Mi. amakusensis View in CoL and Mi. indiana View in CoL , whereas Ac. lordhowensis View in CoL and Mi. pacifica View in CoL are basal to the three species; these have all been placed in Micromussa ( Arrigoni et al., 2016a) View in CoL .
Both our molecular and morphological analyses support the clade grouping these five species ( Fig. 2 View Figure 2 ), whose macromorphological characters are also shared with Acanthastrea regularis Veron, 2000 View in CoL , vol. 3: 16 (Appendix S2). We note that subcorallite morphology and molecular data have not been sampled for the latter species. Superficially, it resembles Favites valenciennesi View in CoL ( Milne Edwards & Haime, 1849b, vol. 12: 124), although possessing thicker walls and more exsert septal teeth. Based parsimoniously on the characters examinable for the holotype, it is clear Ac. regularis View in CoL has no affinity to Acanthastrea View in CoL , and is herein transferred into Micromussa View in CoL . Consequently, the described diversity of this genus currently stands at six species.
Micromussa View in CoL is widely distributed on the reefs of the Indo-Pacific, present from the southern Red Sea ( Arrigoni et al., 2016a) to as far east as the Marshall Islands in the Northern Hemisphere and Fiji in the Southern Hemisphere ( Veron, 2000).
Morphological remarks
Two unambiguous synapomorphies support the Micromussa clade (bootstrap value of 58) – limited coenosteum (likelihood of 0.92 based on the Mk1 model) and strong (pointed) granules on the septal face (likelihood 0.98). Micromussa is the sister genus to Homophyllia based on molecular characters ( Fig. 2A View Figure 2 ), but forms a paraphyletic group with Homophyllia and Australophyllia when analysed using morphological data ( Fig. 2B View Figure 2 ). Micromussa is easily distinguished from these closely related genera by their less numerous septa (24 – 36), costosepta that are not confluent, shorter distance between costa centre clusters (0.3 – 0.6 mm), and the two synapomorphies.
GENUS MOSELEYA QUELCH, 1884: 292 View in CoL ( FIG. 12 View Figure 12 )
Type species
Moseleya latistellata Quelch, 1884: 293 View in CoL ; type by monotypy.
Original description
Corallum compound, flattened, or slightly and broadly convex. Young calicles developing by calicinal marginal budding around a very large median calicle, which has very numerous septal orders, the calicles becoming polygonal and deep at the centre. Epitheca very slight; wall very thin and almost rudimentary, but developed so as to give a distinct simple line of separation to the calicles on the surface, often interrupted, seen in section in a very rudimentary state separating the calicinal centres. Costae very distinct, thin, and finely denticulate. Septa often confluent and continuous from centre to centre in the line of union between adjoining calicles, very thin and close, finely tooth above, and having the teeth subequal or slightly larger near the centre. Endothecal dissepiments vesicular, very abundantly developed, leaving but a very small portion of the septa free exteriorly, seen in transverse section forming nearly concentric lines, and more or less complete tabulae at the centre. A false columella present, seen exteriorly to be formed by the trabeculate and vermiform nature of the innermost upper part of the septa, entirely or almost absent in transverse section, where the septa are seen to meet almost at a point. ( Quelch, 1884: 292 – 293)
Subsequent descriptions
Duncan, 1884: 130 – 131; Quelch, 1886: 110 – 111; Delage & Herouard, 1901: 633; Vaughan & Wells, 1943: 170; Wells, 1955: 6; Wells, 1956: F407; Veron, Pichon & Wijsman-Best, 1977: 201 – 203; Ditlev, 1980: 73; Wood, 1983: 171, 174; Veron, 1986: 534; Chevalier & Beauvais, 1987: 720; Sheppard, 1990: 10; Veron, 1993: 315; Latypov, 1995: 82; Veron, 2000, vol. 3: 269; Latypov, 2006: 174 – 175; Latypov, 2014: 189.
Diagnosis (apomorphies in italics)
Colonial; submassive or massive. Budding intracalicular and extracalicular. Corallites may be polymorphic; discrete. Monticules absent. Walls fused. Calice width large (> 15 mm), with high relief (> 6 mm). Costosepta mostly confluent. Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Paliform (uniaxial) lobes weakly or moderately developed if present. Epitheca reduced. Endotheca usually low – moderate (tabular), but may be abundant ( Fig. 12A, D View Figure 12 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Teeth tall (> 0.6 mm). Tooth spacing medium (0.3 – 1.0 mm), with> 6 teeth per septum. Tooth shape unequal between first- and third-order septa. Tooth size equal between wall and septum. Granules scattered on septal face; irregular in shape. Interarea palisade ( Fig. 12B, E View Figure 12 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines weak. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 12C, F View Figure 12 ).
Species included
Moseleya latistellata Quelch, 1884: 293 View in CoL ; holotype: NHMUK 1886.12.9.158 (dry specimen); type locality: Wednesday Island , Torres Strait, Australia (15 m depth); phylogenetic data: molecular and morphology.
Taxonomic remarks
The genus was established by Quelch (1884: 292) based on material collected from the HMS Challenger expedition at Torres Strait, Australia. It was named in honour of Henry Nottidge Moseley, a British naturalist on the expedition, and placed within a new subfamily Moseleyinae . It is the senior homonym of the grenadier fish Moseleya Goode & Bean, 1895 , named after the same Challenger naturalist, but which has been replaced by Coryphaenoides Gunnerus, 1765 . Moseleya latistellata Quelch, 1884: 293 , remains the only species to have been described in this genus, and is the type by monotypy.
Vaughan & Wells (1943: 170) transferred Moseleya into Faviidae Gregory, 1900 , and subsequent authors have followed suit ( Wells, 1956; Veron et al., 1977; Wood, 1983; Veron, 1986, 2000; Veron & Marsh, 1988). However, the first molecular data for Mos. latistellata presented by Huang et al. (2011) showed that it is nested in the clade XIX + XX ( sensu Fukami et al., 2008 ), later classified as Lobophylliidae Dai & Horng, 2009 . Budd et al. (2012) then formally transferred the genus into Lobophylliidae in the first monograph of the present series. Analyses with expanded taxon sampling have continually supported this classification ( Huang, 2012; Arrigoni et al., 2012, 2014b,c, 2015; Huang & Roy, 2013, 2015; Fig. 2A View Figure 2 ), and so have independent analyses using morphological data ( Huang et al., 2014b; Fig. 2B View Figure 2 ).
Moseleya is restricted to reefs of the central Indo- Pacific between southern Taiwan and northern Australia ( Veron, 2000).
Morphological remarks
There are two autapomorphies that unambiguously define this monotypic genus. Moseleya has fused walls and weakly or moderately developed paliform (uniaxial) lobes, although this is sometimes absent. These traits clearly distinguish Moseleya from the closely related Sclerophyllia , with which it forms a poorly supported clade based on molecular and morphological data. Other characters that are present in Moseleya but not in Sclerophyllia include confluent costosepta, reduced epitheca, and medium tooth spacing (0.3 – 1.0 mm).
Moseleya View in CoL can easily be mistaken for a Pacific ‘faviid’ ( Merulinidae View in CoL ) as it possesses relatively thin walls and costosepta, and has indeed been placed in Faviidae View in CoL since Vaughan & Wells (1943: 170) until as recently as Veron (2000, vol. 3: 269; see also Wells, 1955). However, it possesses several key traits that place it firmly within Lobophylliidae View in CoL , including irregular tooth tip at midcalice that are orientated parallel to the septum, unequal tooth shape between the first- and third-order septa, as well as> 0.6 and> 0.5 mm separating the costa and septum centre clusters, respectively.
GENUS OXYPORA SAVILLE KENT, 1871: 283 View in CoL
( FIG. 13 View Figure 13 )
Synonym
Trachypora Verrill, 1864: 53 View in CoL (type species: Trachypora lacera Verrill, 1864: 53 View in CoL ; original designation, Verrill, 1864: 53); non Trachypora View in CoL Milne Edwards & Haime (1851a, vol. 5: 158).
Type species
Trachypora lacera Verrill, 1864: 53 View in CoL ; subsequent designation, Wells, 1936: 122.
Original description
This name is proposed in place of Trachypora of A. E. Verrill (Bulletin Mus. Comp. Zoology, Cambridge, U. S. p. 53, 1863), which has been already adopted by Milne-Edwards for a genus of the Cyathophylliidae. He separates it from Echinopora on account of the echinate and coarsely costate character of the lower surface of the corallum. ( Saville Kent, 1871: 283 – 284)
Subsequent descriptions
Quelch, 1886: 129; Delage & Herouard, 1901: 641; Yabe & Eguchi, 1935b: 431; Wells, 1936: 122; Yabe et al., 1936: 53; Vaughan & Wells, 1943: 197 – 198; Crossland, 1952: 158; Wells, 1956: F419; Nemenzo, 1959: 121; Chevalier, 1975: 383 – 384; Ditlev, 1980: 81; Veron & Pichon, 1980: 313 – 314; Scheer & Pillai, 1983: 153 – 154; Wood, 1983: 198 – 199; Veron, 1986: 378; Chevalier & Beauvais, 1987: 726; Veron & Hodgson, 1989: 265; Sheppard, 1990: 16; Sheppard & Sheppard, 1991: 109; Latypov & Dautova, 1998: 46; Veron, 2000, vol. 2: 334; Claereboudt, 2006: 206; Latypov, 2006: 330; Latypov, 2014: 340.
Diagnosis
Colonial; laminar. Budding intracalicular. Corallites may be polymorphic; organically united and lacking distinct calical walls. Monticules absent. Coenosteum spinose; extensive amount (≥ corallite diameter). Calice width medium (4 – 15 mm), with low relief (<3 mm). Costosepta mostly confluent. Septa in <3 cycles (<24 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and compact (one to three threads), ≥ 1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes absent. Epitheca absent. Endotheca low – moderate (tabular) ( Fig. 13A, D View Figure 13 ).
Tooth base at midcalice elliptical-parallel. Tooth tip forming multiaxial bulb. Tooth height medium (0.3 – 0.6 mm). Tooth spacing medium (0.3 – 1.0 mm), with ≤ 6 teeth per septum. Tooth size equal between wall and septum. Granules scattered on septal face; weak (rounded). Interarea smooth ( Fig. 13B, E View Figure 13 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits with extensive stereome. Costa centre clusters weak;> 0.6 mm between clusters; medial lines strong. Septum centre clusters weak; 0.3 – 0.5 mm between clusters; medial lines weak ( Fig. 13C, F View Figure 13 ).
Species included
1. Oxypora lacera ( Verrill, 1864: 53) ; syntypes: MCZ IZ 44065, IZ 44066 (two dry specimens); type locality: Singapore; phylogenetic data: molecular and morphology.
2. Oxypora convoluta Veron, 2000 , vol. 2: 340, figs 1 – 4 (see also Veron, 2002: 114, figs 216 – 220; ICZN, 2011: 165); lectotype (designated herein): MTQ G55792 (dry specimen); type locality: Ras Mohammed National Park, Sharm al-Sheikh, Sinai Peninsula, Egypt (20 m depth); phylogenetic data: molecular and morphology.
3. Oxypora crassispinosa Nemenzo, 1979: 12 , pl. 4: fig. 2; holotype: SU CRS-023; type locality: San Plavo Reef , San Carlos City, Negros Occidental, Philippines (18 m depth); phylogenetic data: none .
4. Oxypora egyptensis Veron, 2000 , vol. 2: 341, fig. 5 (see also Veron, 2002: 116, figs 221 – 223; ICZN, 2011: 165); lectotype (designated herein): MTQ G55784 (dry specimen); type locality: eastern Sinai Peninsula, Egypt (15 m depth); phylogenetic data: none.
5. Oxypora glabra Nemenzo, 1959: 122 , pl. 18: fig. 2; holotype: UP C-300 (dry specimen); type locality: Paniquian Island , Puerto Galera, Philippines; phylogenetic data: molecular and morphology .
Taxonomic remarks
Oxypora View in CoL was established by Saville Kent (1871: 283) to replace Trachypora Verrill, 1864: 53 View in CoL , which was represented by Tra. lacera Verrill, 1864: 53 View in CoL , but had already been used by Milne Edwards & Haime (1851a, vol. 5: 158) for a Devonian tabulate coral ( Wells, 1936). Saville Kent’s proposal was probably unknown to Klunzinger (1879), who placed Tra. lacera View in CoL in Echinophyllia Klunzinger, 1879: 69 ( Quelch, 1886) View in CoL . Partly as a result of this affiliation, Oxypora View in CoL was grouped by Wells (1935) with Echinophyllia View in CoL , Tridacophyllia de Blainville, 1830: 327 View in CoL (= Pectinia de Blainville, 1825: 201 View in CoL ), Mycedium View in CoL , and Physophyllia Duncan, 1884: 118 View in CoL , in Tridacophylliidae Thiel, 1932: 96, which was originally placed in Fungida (see Yabe & Eguchi, 1935b). Trachypora lacera View in CoL was later designated as the type of Oxypora View in CoL by Wells (1936), validating it as a separate genus from Echinophyllia View in CoL .
Oxypora View in CoL was placed in the newly established Pectiniidae by Vaughan & Wells (1943: 196), along with the five Tridacophylliidae genera above. Until relatively recently, this classification remained stable (e.g. Wells, 1956; Nemenzo, 1959; Chevalier, 1975; Wood, 1983; Veron, 2000). Molecular-based phylogenies have indicated that Pectinia View in CoL , Mycedium View in CoL , and Physophyllia View in CoL are in the Merulinidae View in CoL clade, distinct from the sister groups comprising Echinophyllia View in CoL and Oxypora View in CoL (subclade F + G sensu Arrigoni et al., 2014c ) that are nested within Lobophylliidae View in CoL (clade XIX sensu Fukami et al., 2008 ; Arrigoni et al., 2014b,c, 2015, 2016a). Consequently, Pectiniidae has been synonymized ( Budd et al., 2012; see also Huang et al., 2011, 2014b; Arrigoni et al., 2012).
Oxypora View in CoL is widely distributed on the reefs of the Indo-Pacific, present from the Red Sea and East Africa to as far east as the Marshall Islands in the Northern Hemisphere and Samoa in the Southern Hemisphere ( Veron, 2000).
Morphological remarks
There are no unambiguous apomorphies for Oxypora , although compact columellae (one to three threads) and the absence of distinct paliform (uniaxial) lobes are synapomorphies on the morphological phylogeny. The three representatives analysed here are nested within the clade dominated by Echinophyllia (subclade F + G sensu Arrigoni et al., 2014c ), as a polyphyletic group on the molecular tree ( Fig. 2A View Figure 2 ), and as a monophyly on the morphological tree ( Fig. 2B View Figure 2 ). Together with Echinomorpha , these genera form a well-supported clade with a bootstrap value of 71 and decay index of 4, and are defined by four synapomorphies: (1) organically united corallites (likelihood of 0.86 based on the Mk1 model); (2) extensive coenosteum (≥ corallite diameter) (likelihood 0.75); (3) columellae ≥ 1/4 of calice width (likelihood 0.92); and (4) loss of epitheca (likelihood 0.84).
Historically, the affiliation between Oxypora and Echinophyllia has been extremely close. The latter was synonymized under the former by Crossland (1952), who found no morphological traits to separate the two genera. Chevalier (1975) also placed Ox. glabra Nemenzo, 1959: 122 , under Echinophyllia based on a specimen from New Caledonia. This resulted in Ox. lacera ( Verrill, 1864: 53) being the sole species classed in Oxypora during that time. Interestingly, the position of Ox. glabra on the molecular phylogeny ( Fig. 2A View Figure 2 ) does show that Ox. glabra is more closely related to all Echinophyllia species except Echinophyllia echinata , which forms a clade with Ox. lacera and Ox. convoluta Veron, 2000 , vol. 2: 340. The close relationship between Echinophyllia and Oxypora is further supported by the presence of alveoli, which are small pits on the exotheca forming at points of insertion of new septocostae ( Chevalier, 1975; Wood, 1983; Veron, 1986, 2000; Benzoni, 2013). As explained above for Echinophyllia , the unexpected split of this group into the molecular clades F and G, not accompanied by consistent morphological variation, indicates that the Echinophyllia – Oxypora dichotomy ought to be tested with more comprehensive taxonomic and genetic sampling of Oxypora .
GENUS SCLEROPHYLLIA KLUNZINGER, 1879: 4 View in CoL
( FIG. 14 View Figure 14 )
Type species
Sclerophyllia margariticola Klunzinger, 1879: 4 View in CoL , pl. 1: fig. 12; type by monotypy.
Original description
Polypar mit sehr entwickelter Epithek, an der Basis breit, aufgewachsen, im Alter nicht frei, nieder, ziemlich breit. Rippen in der N€ ahe des Kelchrandes wohl entwickelt, oben mit einigen Dornchen, weiter herab durch die Epithek ganz verdeckt. Septa debordirend, breit, zahlreich; die grosseren dick, sehr grob und ungleich gez€ ahnt, auch innen und unten. Die Columella hat die Tendenz, compact zu werden. Auch die Interseptalr€ aume der Kelche zeigen die Neigung, sich auszuf ullen € mit compacter Substanz. ( Klunzinger, 1879: 4)
Subsequent descriptions
Delage & Herouard, 1901: 622; Arrigoni et al., 2015: 155.
Diagnosis (apomorphy in italics)
Colonial or solitary; colonies submassive or massive. Budding intracalicular and extracalicular in colonies. Corallites monomorphic; discrete. Monticules absent. Coenosteum spinose; limited amount (includes double wall) in colonies. Calice width large (> 15 mm), with high relief (> 6 mm). Costosepta mostly not confluent. Septa in ≥ 4 cycles (≥ 48 septa). Free septa irregular. Septa spaced <6 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> 3 threads), <1/4 of calice width, and discontinuous amongst adjacent corallites with lamellar linkage. Internal lobes usually absent; paliform (uniaxial) lobes weakly developed if present. Epitheca well developed. Endotheca low – moderate (tabular) ( Fig. 14A, D View Figure 14 ).
Tooth base at midcalice elliptical-parallel. Tooth tip orientation parallel. Teeth tall (> 0.6 mm); widely spaced (> 1 mm), with> 6 teeth per septum. Tooth shape unequal between first- and third-order septa. Tooth size equal between wall and septum. Granules scattered on septal face; irregular in shape. Interarea palisade ( Fig. 14B, E View Figure 14 ).
Walls formed by dominant paratheca and partial septotheca. Thickening deposits in concentric rings with extensive stereome. Costa centre clusters strong;> 0.6 mm between clusters; medial lines weak. Septum centre clusters weak;> 0.5 mm between clusters; medial lines weak ( Fig. 14C, F View Figure 14 ).
Species included
1. Sclerophyllia margariticola Klunzinger, 1879: 4 , pl. 1: fig. 12; lectotype: ZMB Cni 2181; type locality: ‘ Koseir’ (specimen label), Egypt, Red Sea; phylogenetic data: molecular and morphology .
2. Sclerophyllia maxima ( Sheppard & Salm, 1988: 276, figs 4, 5); holotype: NHMUK 1986.11.17.2 (dry specimen); type locality: Muscat, Oman (14 m depth); phylogenetic data: molecular (see also Arrigoni et al., 2015) and morphology.
Taxonomic remarks
The genus was described by Klunzinger (1879: 4) for the solitary and monocentric species Scl. margariticola Klunzinger, 1879: 4 , first collected from the Red Sea in Egypt. It was later found in Djibouti by Gravier (1907, 1911; see also Vaughan, 1907) but, soon after, synonymized under Lobophyllia ( Matthai, 1928) and Symphyllia ( Wells, 1937, 1956; Vaughan & Wells, 1943) as monocentric juvenile stages of these colonial genera. Sclerophyllia , Rhodocyathus Bourne, 1905: 191 , and Protolobophyllia Yabe & Sugiyama, 1935: 381 , were subsequently considered a junior synonym of Cynarina by Wells (1964) and Veron & Pichon (1980). Specifically, they regarded Cyn. lacrymalis ( Milne Edwards & Haime, 1849a, vol. 11: 238) and Scl. margariticola to be the same species.
However, the most recent phylogenetic analyses performed by Arrigoni et al. (2015) and the present study based on both molecular and morphological data ( Fig. 2 View Figure 2 ), have demonstrated that Scl. margariticola is a distinct species most closely related to a species restricted to the Arabian Peninsula, Ac. maxima Sheppard & Salm, 1988: 276 , and not the widespread Cyn. lacrymalis . The monophyly of Scl. margariticola + Ac. maxima , also known as subclade C ( sensu Arrigoni et al., 2014c ), is well supported, and thus Sclerophyllia has been resurrected to incorporate these two species ( Arrigoni et al., 2015).
Sclerophyllia View in CoL is restricted to reefs of the Arabian Peninsula and Arabian Sea ( Sheppard & Sheppard, 1991; Veron, 2000; Arrigoni et al., 2015).
Morphological remarks
The well-developed epitheca is an unambiguous synapomorphy (likelihood of 1.00 based on the Mk1 model) recovered for the Sclerophyllia clade. The two members of this genus share all the micromorphological characteristics analysed here, including those illustrated by Arrigoni et al. (2015), i.e. high elliptical septal teeth parallel to the septum, irregular lobate tips, wide tooth spacing (> 1 mm), granules scattered on the septal face, and a palisade interarea.
Sclerophyllia is closely related to Moseleya . They form a monophyletic group on the morphological tree and a paraphyletic grade on the molecular tree ( Fig. 2 View Figure 2 ). However, they are separated based on the more common presence of weak to moderate paliform lobes, reduced epitheca, and smaller tooth spacing in Moseleya . Monostomatous Sclerophyllia specimens are always of the species Scl. margariticola . The only other lobophylliid taxon that is exclusively monostomatous is Cynarina .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dipsastraea faviaformis ( Veron, 2000 )
| Huang, Danwei, Arrigoni, Roberto, Benzoni, Francesca, Fukami, Hironobu, Knowlton, Nancy, Smith, Nathan D., Stolarski, Jarosław, Chou, Loke Ming & Budd, Ann F. 2016 |
Echinophyllia nishihirai
| Veron JEN 1990: 130 |
Echinomorpha nishihirai
| Veron JEN 1990: 130 |
Acanthastrea amakusensis
| Veron JEN 1990: 137 |
Australomussa
| Veron JEN 1985: 171 |
| Veron JEN 1985: 171 |
| Veron JEN 1985: 171 |
| Wells JW 1964: 379 |
| Wells JW 1964: 379 |
| Yabe H & Sugiyama T & Eguchi M 1936: 44 |
| Yabe H & Sugiyama T & Eguchi M 1936: 44 |
| Yabe H & Sugiyama T & Eguchi M 1936: 44 |
| Matthai G 1928: 229 |
| Dana JD 1846: 187 |
| Dana JD 1846: 186 |
| Quoy JRC & Gaimard JP 1833: 227 |
Symphyllia wilsoni
| Veron JEN 1985: 167 |
Acanthophyllia
| Wells JW 1937: 242 |
| Wells JW 1937: 242 |
| Yabe H & Sugiyama T 1935: 381 |
| Yabe H & Sugiyama T 1935: 382 |
| Yabe H & Sugiyama T 1931: 128 |
| Bourne GC 1905: 191 |
| Bourne GC 1905: 191 |
| Bourne GC 1905: 191 |
| Michelin H 1850: 238 |
Acanthophyllia
| Wells JW 1937: 242 |
Oxyphyllia
| Yabe H & Eguchi M 1935: 377 |
| Yabe H & Eguchi M 1935: 377 |
| Ellis J & Solander DC 1786: 156 |
MOSELEYA QUELCH, 1884: 292
| Quelch JJ 1884: 292 |
Moseleya latistellata
| Quelch JJ 1884: 293 |
Moseleya latistellata
| Quelch JJ 1884: 293 |
ECHINOPHYLLIA KLUNZINGER, 1879: 69
| Klunzinger CB 1879: 69 |
SCLEROPHYLLIA KLUNZINGER, 1879: 4
| Klunzinger CB 1879: 4 |
Sclerophyllia margariticola
| Klunzinger CB 1879: 4 |
OXYPORA SAVILLE KENT, 1871: 283
| Saville Kent W 1871: 283 |
Oxypora
| Thiel ME 1932: 96 |
| Duncan PM 1884: 118 |
| Quelch JJ 1879: 69 |
| Saville Kent W 1871: 283 |
| Verrill AE 1864: 53 |
| Verrill AE 1864: 53 |
| de Blainville HMD 1830: 327 |
| de Blainville HMD 1825: 201 |
Trachypora
| Verrill AE 1864: 53 |
| Verrill AE 1864: 53 |
| Verrill AE 1864: 53 |
Trachypora lacera
| Wells JW 1936: 122 |
| Verrill AE 1864: 53 |
LOBOPHYLLIA DE BLAINVILLE, 1830: 321
| de Blainville HMD 1830: 321 |
Lobophyllia
| Matthai G 1928: 208 |
| Dana JD 1846: 40 |
| Dana JD 1846: 179 |
| Ehrenberg CG 1834: 325 |
| Quoy JRC & Gaimard JP 1833: 195 |
| de Blainville HMD 1830: 321 |
| Cairns SD 1829: 93 |
| De Chamisso A & Eysenhardt CG 1821: 369 |
| Lamarck JBP 1816: 229 |
| Oken L 1815: 73 |
| Ellis J & Solander DC 1786: 153 |
| Forskal P 1775: 137 |
| Forskal P 1775: 137 |
| Pallas PS 1766: 299 |
| Pallas PS 1766: 301 |
Madrepora aspera
| Wells JW 1936: 111 |
| Ellis J & Solander DC 1786: 156 |
Madrepora corymbosa
| Matthai G 1928: 210 |
| Forskal P 1775: 137 |