Bipalium vagum, Jones & Sterrer, 2005
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1001.1.3 |
|
DOI |
https://doi.org/10.5281/zenodo.10532394 |
|
persistent identifier |
https://treatment.plazi.org/id/F36987E4-226D-970D-FEE5-58EEFC618396 |
|
treatment provided by |
Felipe |
|
scientific name |
Bipalium vagum |
| status |
sp. nov. |
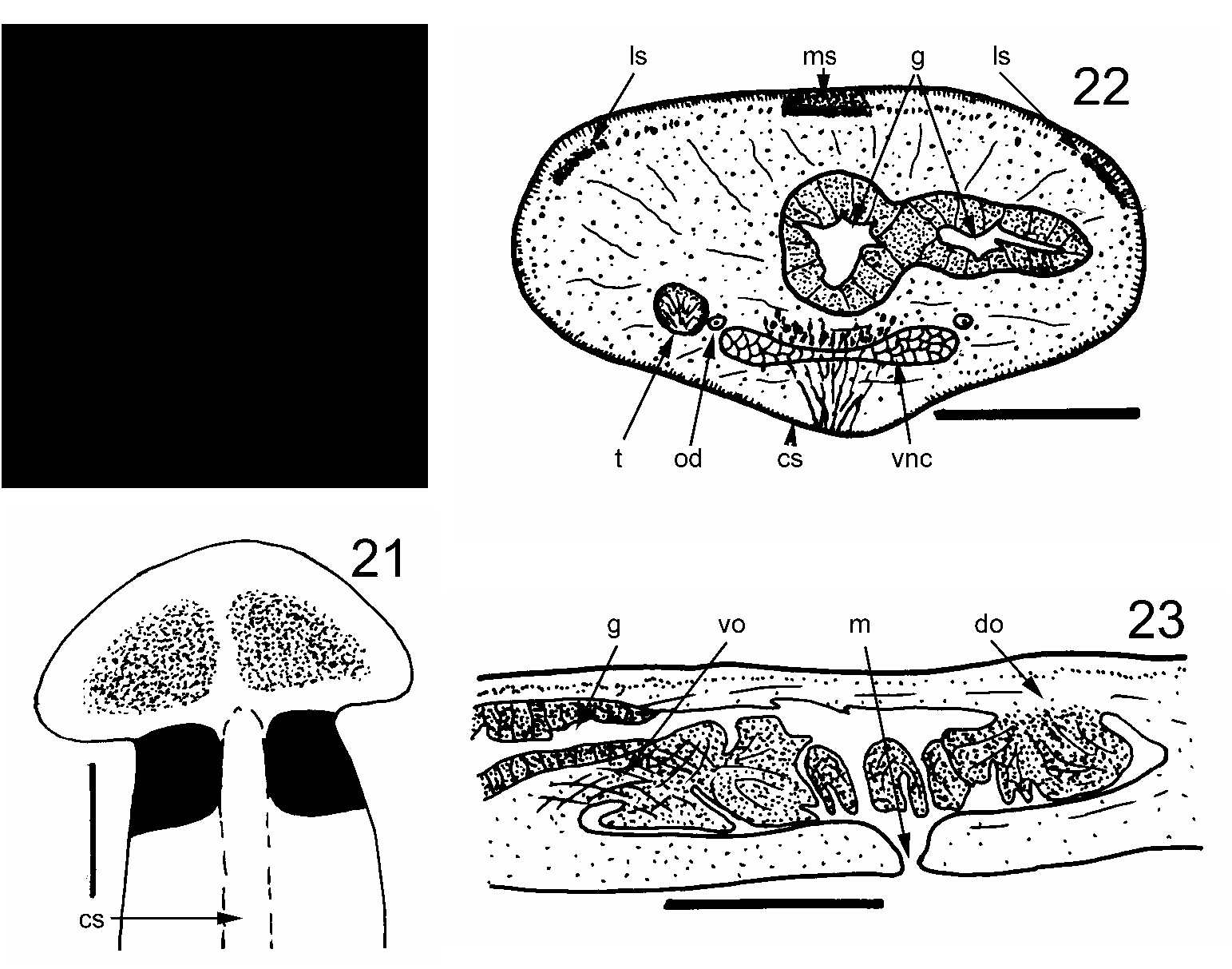
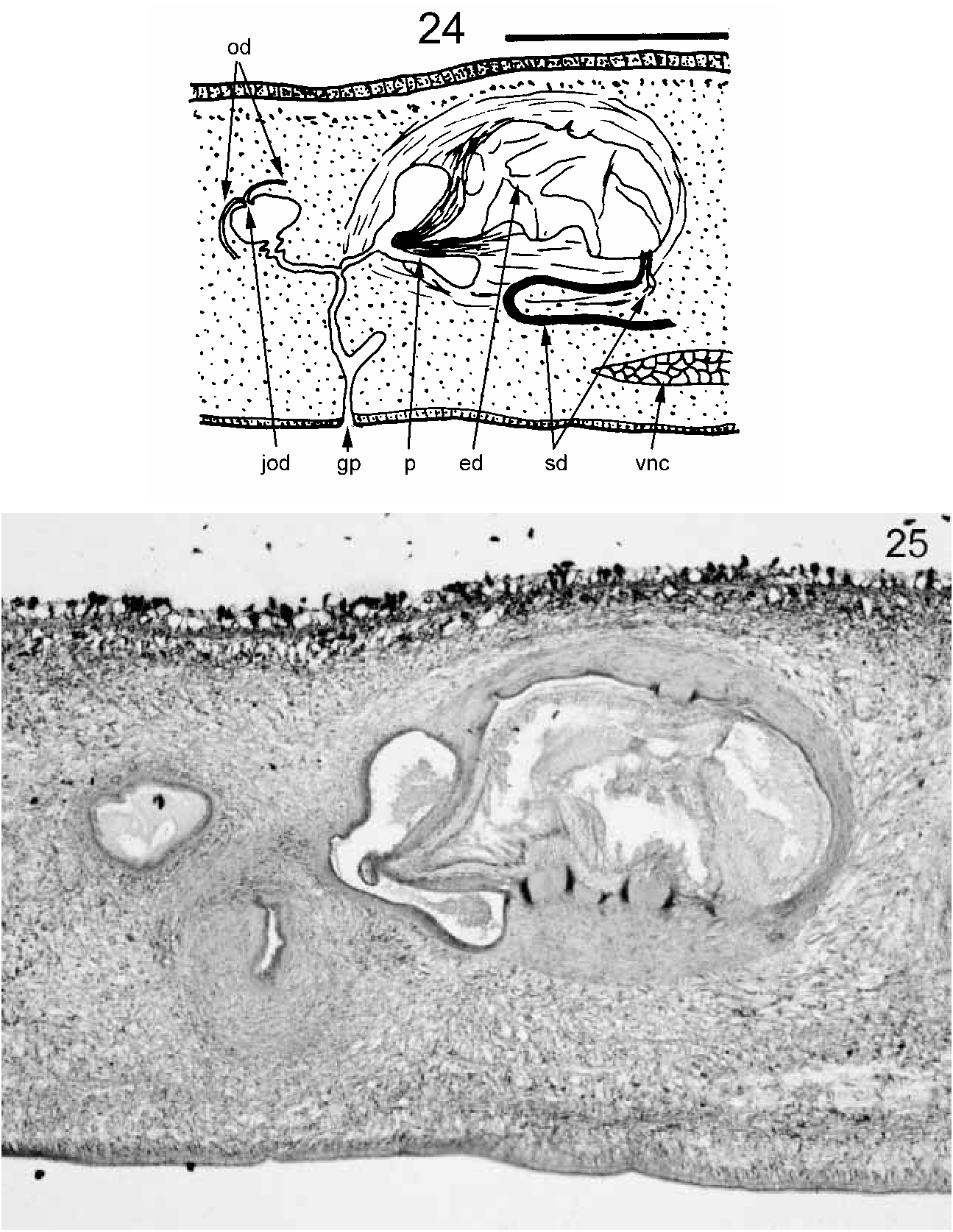
Bipalium vagum n. sp. ( Figs 20–25 View FIGURES 20–23 View FIGURES 24–25 , Color Plate 5)
Material examined: Holotype. Divided by transverse cuts into five portions. Portions containing the pharynx (5 slides) and copulatory apparatus (3 slides) sectioned longitudinally. Small portion anterior to the pharynx sectioned transversely (1 slide). Remaining anterior and posterior portions preserved in 80% alcohol. Deposited in the Natural History Museum, London. Accession number 2004.12.8.16.
Paratypes: Three further whole specimens preserved in 80% alcohol. Deposited in the Natural History Museum, London. Accession numbers 2004.12.8.17–19.
Type locality: Spanish Point, Pembroke Parish, Bermuda.
Etymology: From vagum , meaning wandering or vagrant, recognizing that this species certainly originated elsewhere.
Description: The first specimens of this species were found by HDJ in February 2003 at Aberfeldy Nursery, Paget Parish, Bermuda. It has subsequently been found in several localities on the islands. A specimen with a visible gonopore, found at Spanish Point, Pembroke Parish, was selected as the holotype. Its preserved dimensions were: length 24 mm; width about 1.5 mm; height about 0.75 mm. The pharyngeal aperture (mouth) and gonopore were respectively 12 mm (50%) and 17 mm (71%) from the anterior end. Lengths (preserved) of further specimens range from 14 to 22 mm. The head is laterally expanded but not recurved posteriorly ( Figs 20, 21 View FIGURES 20–23 ; Color Plate 5). The head is dark brown marginally but there are two large black patches separated by the lighter buff ground colour. Eyes are numerous and distributed round the margin of the head and neck. The neck is slightly narrower than the remainder of the body which has parallel margins. There is a black band at the neck which continues laterally and ventrally and stops at the ventral creeping sole ( Figs 20, 21 View FIGURES 20–23 ). In the midline a narrow extension of the black pigmentation runs forwards and almost fuses with the two black head patches. Dorsally, there are three longitudinal dark stripes on the pale brown ground colour, a broad, middorsal and sharply demarcated black stripe and two lateral broad dark brown stripes, less sharply demarcated, all starting at the transverse neck band and continuing the full length of the flatworm. The dorsal ground colour and ventral side are pale brown. The creeping sole forms a midventral raised ridge, pale and about one quarter of the body width wide ( Figs 21, 22 View FIGURES 20–23 ). The dorsal epidermis is about 40 µm thick. Rhabdites are sparse, though two types are present. Long, extremely thin sinuous ones and short wider ones which are less numerous. Cilia are not discernible. The epidermis is thinner laterally and ventrally lateral to the creeping sole. The epidermis of the creeping sole is about 20 µm thick, contains no rhabdites and is densely ciliated, except for the very midline. The cilia are about 5 µm long. Nuclei are basal. Longnecked cyanophilic glands discharge near the nonciliated centreline of the sole. The cell bodies of these glands appear to be dorsal to the ventral nerve cords. The subepidermal muscle layer is thin dorsally, less than the thickness of the epidermis – about 30 µm, and consists of circular muscle fibres and very small bundles of longitudinal muscle fibres not clearly defined. Pigment granules of the black middorsal stripe and brown lateral stripes are present amongst these subepidermal muscles. There appear to be no subcutaneous muscles above the epithelium of the creeping sole. Thus CMI is 1.6%. Other muscle fibres in various orientations are distributed through the body but form no clear muscular layer. Much of the body is filled with material that may be parenchymatous or vitelline glandular tissue. The nervous system in transverse section consists of a pair of ventral nerve cords joined by transverse commissures ( Fig. 22 View FIGURES 20–23 ) and with lateral extensions. The pharynx is much folded and plicate or “kragenförmig” (collarform), the dorsal origin being towards the rear of the pharyngeal pouch ( Fig. 23 View FIGURES 20–23 ). Presumably there is a pair of ovaries anteriorly (this would require sectioning to confirm). However, a pair of ovovitelline ducts run dorsolaterally to the ventral nerve cord on either side ( Fig. 22 View FIGURES 20–23 ). The ovovitelline ducts run posterior to the gonopore, turn dorsally, unite and immediately discharge into a small cavity ( Fig. 24 View FIGURES 24–25 ). This cavity opens via a narrow duct (?female antrum) is joined by a similar duct from the penis, and opens to the gonopore. Testes ( Fig. 22 View FIGURES 20–23 ) are numerous, ventral and lateral to the ventral nerve cords. They appear to stop anterior to the pharynx. Sperm ducts are not discernible for most of their length, but probably run dorsal to each ventral nerve cord. Ventral to the copulatory apparatus, each sperm duct expands and becomes convoluted to form a sperm storage organ ( Fig. 24 View FIGURES 24–25 ). These two ducts turn dorsally and forwards then dorsally and separately discharge close together through very narrow ducts into the anterior base of the large, bulbous ejaculatory cavity within the penis ( Figs 24, 25 View FIGURES 24–25 ). This bulbous cavity is surrounded by a layer of musculature, most fibres running round the bulb. The cavity discharges posteriorly through a short, thinwalled conical penis papilla ( Figs 24, 25 View FIGURES 24–25 ). The musculature of the penis papilla is dense and consists of an outer layer of circular fibres about 20 µm thick, and an inner layer of longitudinal fibres about 30 µm thick. The penis opens into the male antrum which joins the female antrum in a small cavity via a narrow duct to form a narrow common antrum leading to the gonopore.
Discussion: For bipaliid species where the copulatory apparatus is known (as in the Bermuda species), Ogren & Sluys (1998) tentatively grouped bipaliids according to four characters of the copulatory apparatus. The Bermuda species has the following characters (definitions of abbreviations given in Ogren & Sluys 1998): female organ is vertical (FCA 1); ovovitelline duct approaches the female canal from the posterior (OVD 0); penis papilla of the fixed conical type enclosing a large part of the ejaculatory duct (PPT 0); Male antrum wall is muscular and forms a prominent narrow penis sheath separating the male antrum from the common antrum (MAW 1). These are the characters of the Bipalium group as defined by Ogren & Sluys (1998). The genus Novibipalium was proposed by Kawakatsu et al. (1998) for those bipaliid species with a reduced penis papilla and an elongate penis sheath (pseudophallus). The Bermuda specimen has a penis papilla thus does not fit that definition. The genus Humbertium was erected by Ogren & Sluys (2001) for Groups B1 and B2 of Ogren & Sluys (1998). Humbertium includes species with ovovitelline ducts that turn dorsally anterior to the level of the gonopore. This is not the case in the Bermuda specimens. Bipaliid species incompletely described or described on external characters only are placed in the collective genus Diversibipalium ( Kawakatsu et al. 2002) . External characters could be used to identify the Bermuda specimens with such species. Graff (1899), in his identification key, lists 14 bipaliid species with three dark dorsal stripes. The Bermuda specimens match none of these. Ogren (1987) lists a further seven species with three dark dorsal stripes. The only species with three stripes beginning at a darkly pigmented neck (as in the Bermuda specimens) is D. keshavi ( Saxena 1957) . The original description by Saxena (1957) is extremely brief, 16 lines in total, with no illustrations and is of external characters only. Diversibipalium keshavi is described as light brown to grey in colour, 80–200 mm long and 3–5 mm wide. A median dorsal and two lateral dark stripes (no information as to colour or relative width) extend from the neck which is darkly pigmented (again, no information as to colour). Thus, though the Bermuda specimens could belong to D. keshavi , the description of the latter is inadequate. Further, the Bermuda specimens are fully mature yet much shorter than the reported length of D. keshavi so it seems unlikely that they are of that species. Thus we place the Bermuda specimen in the genus Bipalium as currently defined, hence Bipalium vagum . Certainly the copulatory apparatus is similar in its general characteristics to that of B. kewense (see Winsor 1983).
Specimens of B. vagum have been observed by WS feeding on snails. Again it is presumed that reproduction is by copulation and subsequent laying of egg capsules. It is possible that some asexual reproduction by fission may occur as in some other Bipalium spp. ( Winsor 1983) . The species is certainly introduced to Bermuda. Bipaliidae originate in southeast Asia, India and Madagascar ( Ogren et al. 1997).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.