Theretra shendurneensis, Kunte, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4323.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:0Fdcf96A-3255-4Cf1-878D-4703D07A5A41 |
|
DOI |
https://doi.org/10.5281/zenodo.6038411 |
|
persistent identifier |
https://treatment.plazi.org/id/F375987F-4F0E-FF9E-72B4-3E57FC0B17E9 |
|
treatment provided by |
Plazi |
|
scientific name |
Theretra shendurneensis |
| status |
sp. nov. |
Theretra shendurneensis sp. nov.
( Figs. 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 , Table 1)
ZooBank LSID urn:lsid:zoobank.org:act: 369A10C8-1E5F-41E5-B555-8DA213315372
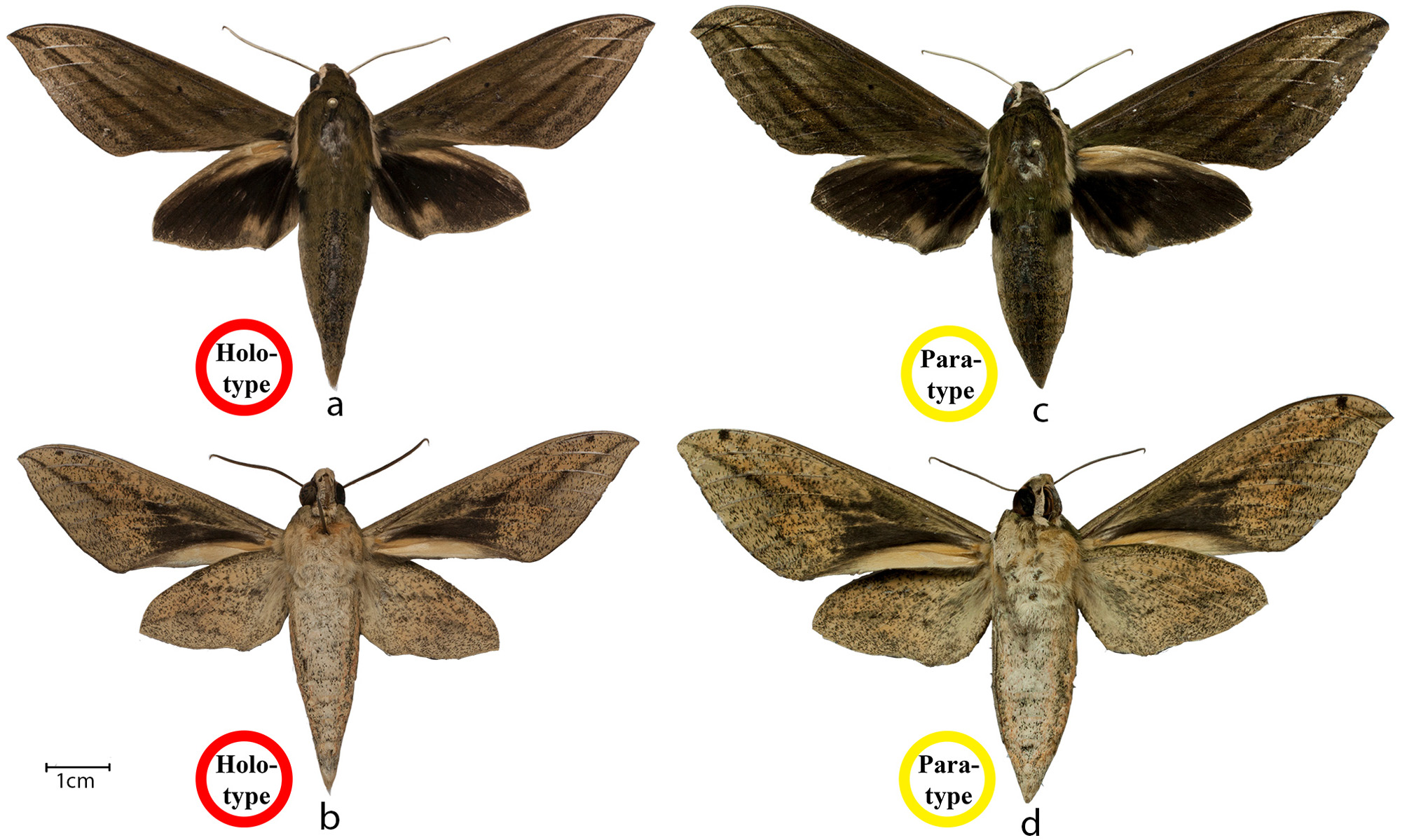
Holotype ( Fig. 4a, b View FIGURE 4 , 5a,b View FIGURE 5 ): ♂, India, Kerala, Kollam District, Shendurney Wildlife Sanctuary, Pandimotta forest camp, 8°49ʹ39″N 77°13ʹ1″E, 1,171 m, 31.v.2014, Yash Sondhi leg. Preserved dry, pinned, deposited in the Research Collections at the National Centre for Biological Sciences, Bengaluru (= Bangalore), India. Voucher code NCBS-QA070 .
Paratype: 1♀ ( Fig. 4c, d View FIGURE 4 , 5c View FIGURE 5 ), data as holotype. Preserved dry, pinned, deposited in the Research Collections at the National Centre for Biological Sciences, Bengaluru, India. Voucher code NCBS – QA587 View Materials . Additional material (non-type): ♂ ( Fig. 5d, e View FIGURE 5 ), data as holotype but date 1.vi.2014 . Only DNA extracted from legs retained from this specimen, deposited in the Research Collections at the National Centre for Biological Sciences, Bengaluru, India. Voucher code NCBS – QA627 View Materials . ♂ ( Fig. 5f View FIGURE 5 ), data as holotype but date 2.vi.2014 (not collected).
Description. Male (holotype; NCBS–QA070): Forewing length: 43.5 mm. Upperside: Head, thorax and abdomen dull greenish-brown dorsally; antennae fasciculate, apically hooked, white scaled above; eyes ringed with brown; frons green; gena and labial palps white; proboscis dark to pale brown; tegulae edged in off-white; abdomen with a pair of basal, lateral black patches and a blackish dorsal stripe flanked by two more diffuse longitudinal lines. Forewing: ground colour dull greenish-brown; discal spot distinct, small, black; antemedial lines two, faint; medial lines three, basal line stronger, almost twice width of middle line, distalmost line very faint; postmedial lines also two, basal line strong, broader and darker than basal antemedial line, narrowing towards apex, distal line weaker, area between them darker than ground colour; submarginal line well-developed, running parallel to termen. Hindwing: ground colour black; a small yellow patch near anal angle with a distinct V-shaped marking; a narrow yellow band running along inner margin, not extending to base; a broad yellow patch extending from base to middle of costa, where it narrows sharply, vein Sc+R highlighted with black within this patch; a small, scaleless region present in the centre of the costal yellow patch in the male only.
Underside. Head, thorax, legs and abdomen pale fawn. Ground colour of both wings pale yellow, faintly irrorated with minute black dots throughout. Forewing: basal half black; costa with a prominent subapical black spot; postmedial line black, concave, slightly sinuous, running from apex to inner margin, sharply angled outwards toward termen at vein Rs3, and irregular from vein Rs3 to inner margin; area distal to postmedial line paler; a pale yellow area along basal half of inner margin. Hindwing: antemedial line faint, formed from a series of oblique vshaped marks; postmedial line even less distinct, curved, running from apex and ending before inner margin.
Female (paratype). Similar to male but larger (forewing length = 51mm); antennae thinner, simple; abdomen bulkier, dorsal stripe fainter; wing pattern more prominent; forewing broader and more rounded in outline.
Male genitalia. Similar to other taxa of the Theretra boisduvalii species-group ( Fig. 6 View FIGURE 6 ): uncus and gnathos forming the typical macroglossine “bird-beak” structure; saccus broad and anteriorly rounded; valve sole-shaped, inner surface covered with strong, basally-directed setae; harpe a low, broadly triangular lobe with indistinct teeth along the distal edge; phallus slightly curved.
DNA barcode. The 648 bp barcode region of COI of the paratype female is deposited in NCBI GenBank (accession number KY688373 View Materials ).
Etymology. The moth is named after the type locality, Shendurney Wildlife Sanctuary.
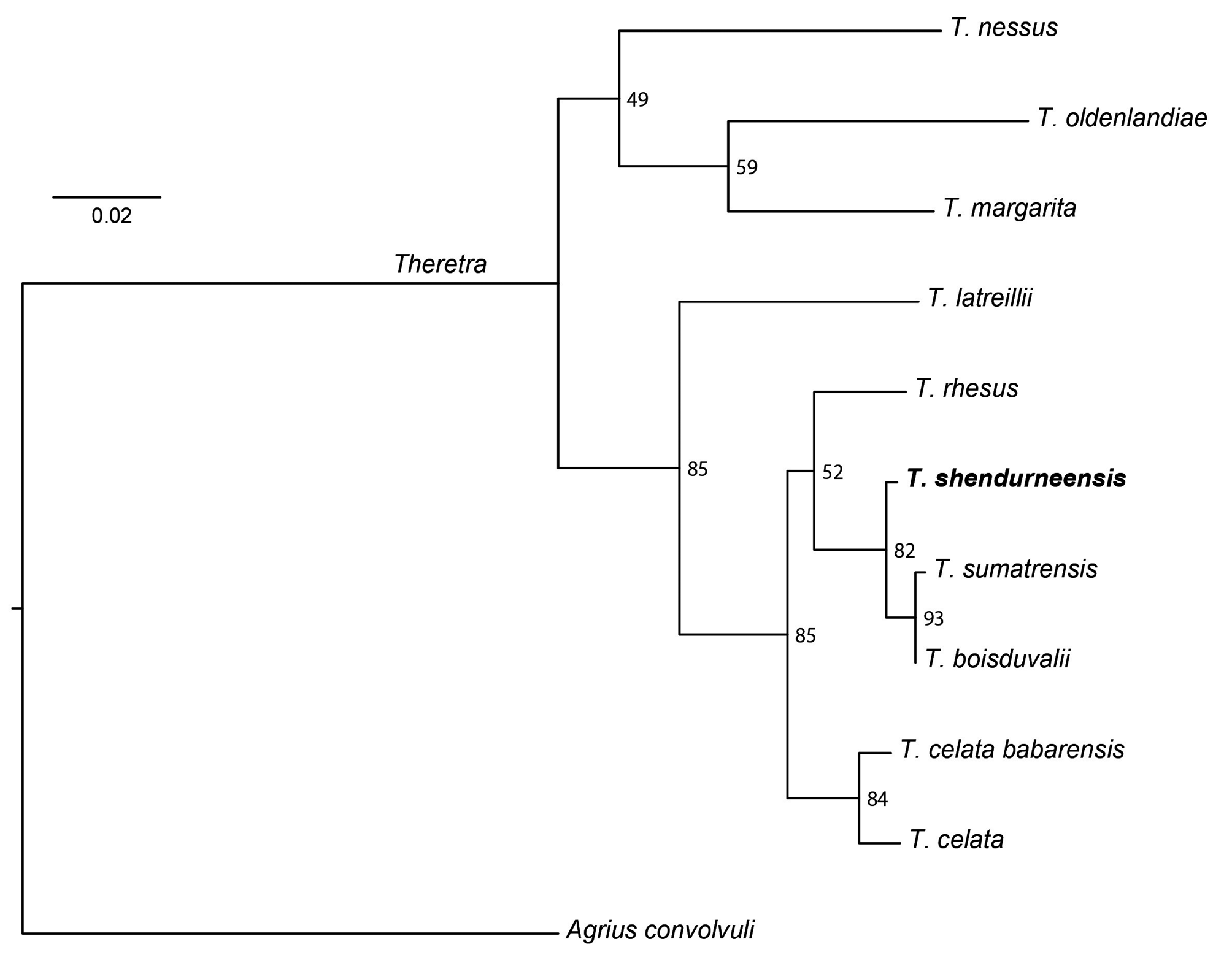
Differential diagnosis. The preliminary DNA barcode analysis undertaken in the present study showed that two other Indian species of Theretra , T. boisduvalii and T. sumatrensis , are closely related to the new species ( Fig. 3 View FIGURE 3 ), although neither has yet been found in the Western Ghats. A third species, T. rhesus , is genetically more distantly related to T. shendurneensis sp. nov. but has a similar habitus; it has not yet been reported from India.
Habitus ( Fig. 7 View FIGURE 7 ): In body and wing pattern T. shendurneensis sp. nov. most closely resembles T. rhesus . However, the two species can be separated by the pattern of oblique lines on the forewing. In T. shendurneensis sp. nov., the distalmost of the three medial lines is barely visible and easily overlooked, whereas in T. rhesus the middle and distal lines are of approximately the same intensity. T. rhesus is also slightly smaller than T. shendurneensis sp. nov. In comparison to these two species, T. boisduvalii and T. sumatrensis are much plainer moths. In both these species, all the oblique lines are much weaker; in particular, the basalmost postmedial line is very faint, only being strongly marked on the veins as a series of dark dots or short dashes. When fresh, T. boisduvalii is a rather greenish moth with a rather uniform suffusion of darker scales, whereas T. sumatrensis is paler and more brownish, with a cleaner pattern, and most specimens have a distinct diffuse darker cloud across the middle of the forewing.
Male genitalia ( Fig. 8 View FIGURE 8 ): The structures of the male genitalia of the four related species are very similar overall. Although the shapes of the uncus, gnathos and saccus show small differences in shape, the harpes are distinctive. The harpe of T. rhesus is distinctly higher than wide, where those of the other three species are at least as wide as high and usually wider. The distal margin of the harpe in T. boisduvalii and T. sumatrensis is convex, rather rounded and broad in the former and more angular and toothed in the latter. In contrast, the distal margin of the harpe in T. shendurneensis is almost straight with minute teeth.
Geographic Distribution. Theretra shendurneensis sp. nov. has so far been reported only from the type locality. There are no historical records ( Bell & Scott 1937) or specimens in the collections of the Natural History Museum, London, that might correspond to this species (I. Kitching, pers. obs.). There are no other known records from Indian museums or from other hawkmoth researchers in India (P.R. Shashank, IRAI, pers. comm.; also personal correspondence and museum visits of Yash Sondhi). A report on the Sphingidae of Karnataka state undertaken by the staff of the Sphingidae Museum, Příbram, Czech Republic ( Melichar, 2012) did not list any records of a species resembling T. shendurneensis sp. nov. Such a narrow distribution is unusual for Theretra , since most other related species appear to be more widespread. The geographical ranges of T. boisduvalii , T. sumatrensis and T. rhesus were modelled as part of a larger macroecological study on Old World Sphingidae by Ballesteros- Mejia et al. (2016) and maps can be found on the Map of Life at https://mol.org/species/ Theretra _ boisduvalii , https://mol.org/species/ Theretra _ sumatrensis and https://mol.org/species/ Theretra _ rhesus respectively. Despite being closest in wing pattern, T. rhesus is restricted to Southeast Asia, from southern Thailand (whence it is predicted to occur in neighbouring parts of Myanmar, Cambodia and Vietnam), Peninsular Malaysia, Sumatra and Java, the Western Lesser Sunda Islands, Borneo, Sulawesi, the Philippines, and Lanyu Island ( Taiwan). It has not been recorded in India, nor is it currently predicted to. Theretra sumatrensis occurs from SW China (Yunnan and Xizang) to central Myanmar, northern Thailand, Laos, Vietnam, Peninsular Malaysia, Java, Sumatra, the Western Lesser Sundas, and Borneo, but it does not reach Sulawesi, the Philippines or Taiwan. It was previously not reported from India, but we here extend its range into NE India based on a specimen from Mawkisyiem, East Khasi Hills District, Meghalaya (NCBS–PW607, male, Fig. 7c View FIGURE 7 ) and two specimens photographed at Langka, Pakke Tiger Reserve, East Kameng District, and the Sessa Orchid Sanctuary, West Kameng District, Arunachal Pradesh (Moths of India image codes bh543 and bk466, respectively; Sondhi et al. 2017). In the Natural History Museum, London, there are five specimens labelled simply “ Sikkim ”, three labelled “Darjeeling”/“Darjiling”, three from “Khasia Hills” and three from “Lakhimpur, Assam ”, all of which were probably collected in the last two decades of the nineteenth century. Theretra boisduvalii has a similar range to T. sumatrensis but extends further east through southern China to Taiwan, and further west though the southern Himalaya to NW India (Himachal Pradesh). Theretra boisduvalii was described from a specimen purportedly from Crete but this is most likely an error. Ballesteros-Mejia et al. (2016) also predicted T. boisduvalii to occur in the north-eastern part of peninsular India, which may be correct but has yet to be confirmed, and crucially in the Western Ghats and Sri Lanka. There were then, and still are, no specimen records to back up this purported range extension, which was based solely on the macroecological models they used. In retrospect, however, it would seem reasonable to infer that this part of the modelled distribution of T. boisduvalii actually represents the predicted distribution of T. shendurneensis sp. nov. Thus, T. shendurneensis sp. nov. may be expected to be more widely distributed in the Western Ghats and possibly also in Sri Lanka and/or other parts of peninsular India such as the Eastern Ghats.
Flight Period. The first author conducted 40 days of field surveys between 2013 and 2016 in the southern Western Ghats during most months (February, April, May, June, August, September, October and November) of the year. The new species was only recorded on 31 May 2014 and 1–2 June 2014, on consecutive days at the same locality (no surveys were undertaken there in 2013 or 2015 and no specimens were recorded in 2016). Hence, as far as known, T. shendurneensis sp. nov. is possibly univoltine, and that adults fly during the summer and premonsoon months.
Status, habitat, and habits. T. shendurneensis sp. nov. appears to be rare, at least at light; however further sampling is necessary to confirm this. Despite extensive collecting and rearing, Bell & Scott (1937) did not find the species, nor did a more recent survey of the hawkmoths of Karnataka ( Melichar, 2012). Even during the surveys of the first author, when T. shendurneensis sp. nov. was seen on three consecutive nights, only four individuals were recorded. The type locality of Pandimotta (8°49ʹ39″ N 77°13ʹ1″ E, elevation 1171 m, Figs 1-2 View FIGURE 1 View FIGURE 2 ), is part of the Shendurney Wildlife Sanctuary (8°48ʹ–8°58ʹ N, 77°4ʹ–77°7ʹ E), which itself is located in the Agasthyamalai Biosphere Reserve in the Thenmala Forest Division of Kollam District of Kerala. The Shendurney Wildlife Sanctuary lies on either side of the Shendurney River and is a biodiverse area from where numerous rare and endemic species of flora and fauna have been recorded ( Sasidharan & Anto 1997; Mathew et al. 2004; Abraham et al. 2013; Joshi et al. 2016). The habitat at Pandimotta comprises mid-elevation evergreen forests and grasslands. Common woody plant genera in this area include Mesua, Hopea, Calophyllum , Cullenia, Syzygium, Cinnamomum, Calamus and Strobilanthus (Birdlife International, 2017) . The vegetation immediately surrounding the Pandimotta forest rest house, where the specimens of this species were collected and photographed, is mid-elevation evergreen forests mixed with reed ( Ochlandra , Poaceae ) patches. A list of all the Sphingidae species collected at the type locality is included in Table 3. The habits of T. shendurneensis sp. nov. are unknown, apart from the fact that it comes to blacklight at night.
Life History. Unknown, but likely to be similar to related Theretra species, which are themselves poorly recorded. The immature stages of T. rhesus are still unknown, and while Dupont & Roepke (1941), and Roessler & Küppers (1977) and Diehl ([1982]), referred to the life history of “ Theretra boisduvalii ”, in Java and Sumatra respectively, these data may refer to T. sumatrensis instead as these two species have been confused in the past. Larvae of the Theretra clotho species group are known to feed mostly on species of plants from the following families: Vitaceae , Araceae , Dilleniaceae and more rarely Malvaceae , Onagraceae and Begoniaceae . The larvae of T. shendurneensis sp. nov. should be searched for, and will probably be found, on such plant families.
Subfamily Species
Macroglossinae Acosmeryx akanshi Melichar, Řezáč, Manjunatha & Horecký, 2014 Macroglossinae Acosmeryx anceus (Stoll, 1781) View in CoL
Macroglossinae Hippotion velox (Fabricius, 1793) View in CoL
Macroglossinae Hippotion rosetta (Swinhoe, 1892) Macroglossinae Hippotion celerio (Linnaeus, 1758) Macroglossinae Rhagastis castor (Walker, 1856)
Macroglossinae Theretra clotho (Drury, 1773)
Macroglossinae Theretra nessus (Drury, 1773)
Macroglossinae Theretra gnoma (Fabricius, 1775)
Macroglossinae Theretra castanea (Moore, 1872)
Macroglossinae Theretra shendurneensis sp. nov.
Macroglossinae Theretra pallicosta (Walker, 1856) Smerinthinae Ambulyx substrigilis Westwood, 1847 Smerinthinae Ambulyx moorei Moore, 1858
Smerinthinae Ambulyx belli (Jordan, 1923)
Smerinthinae Amplypterus panopus (Cramer, 1779) Smerinthinae Marumba nympha Rothschild & Jordan, 1903 Sphinginae Acherontia lachesis (Fabricius, 1798) Sphinginae Agrius convolvuli (Linnaeus, 1758) Sphinginae Megacorma obliqua (Walker, 1856) Sphinginae Meganoton nyctiphanes (Walker, 1856) Sphinginae Psilogramma vates (Butler, 1875)
Sphinginae View in CoL Dolbina manjunatha Haxaire and Melichar 2013
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Theretra shendurneensis
| Kunte, Krushnamegh 2017 |
Acosmeryx akanshi Melichar, Řezáč, Manjunatha & Horecký, 2014
| Melichar, Rezac, Manjunatha & Horecky 2014 |
Dolbina manjunatha
| Haxaire and Melichar 2013 |
Sphinginae
| Latreille 1802 |
Hippotion velox
| Fabricius 1793 |
Acosmeryx anceus
| Stoll 1781 |