Austroconops mcmillani Wirth and Lee
|
publication ID |
https://doi.org/ 10.1206/0003-0082(2004)449<0001:AWALAL>2.0.CO;2 |
|
DOI |
https://doi.org/10.5281/zenodo.5060651 |
|
persistent identifier |
https://treatment.plazi.org/id/F966E84E-FFB4-215F-FF4A-FCAEFEE10249 |
|
treatment provided by |
Felipe |
|
scientific name |
Austroconops mcmillani Wirth and Lee |
| status |
|
Austroconops mcmillani Wirth and Lee View in CoL
Austroconops mcmillani Wirth and Lee, 1958: 337 View in CoL . Holotype female, National Park , Perth, Western Australia, Australia, 12XII1954 (ANIC).
Austroconops mcmillani: Borkent, Wirth and Dyce, 1987 View in CoL . Male adult. Female adult (in part).
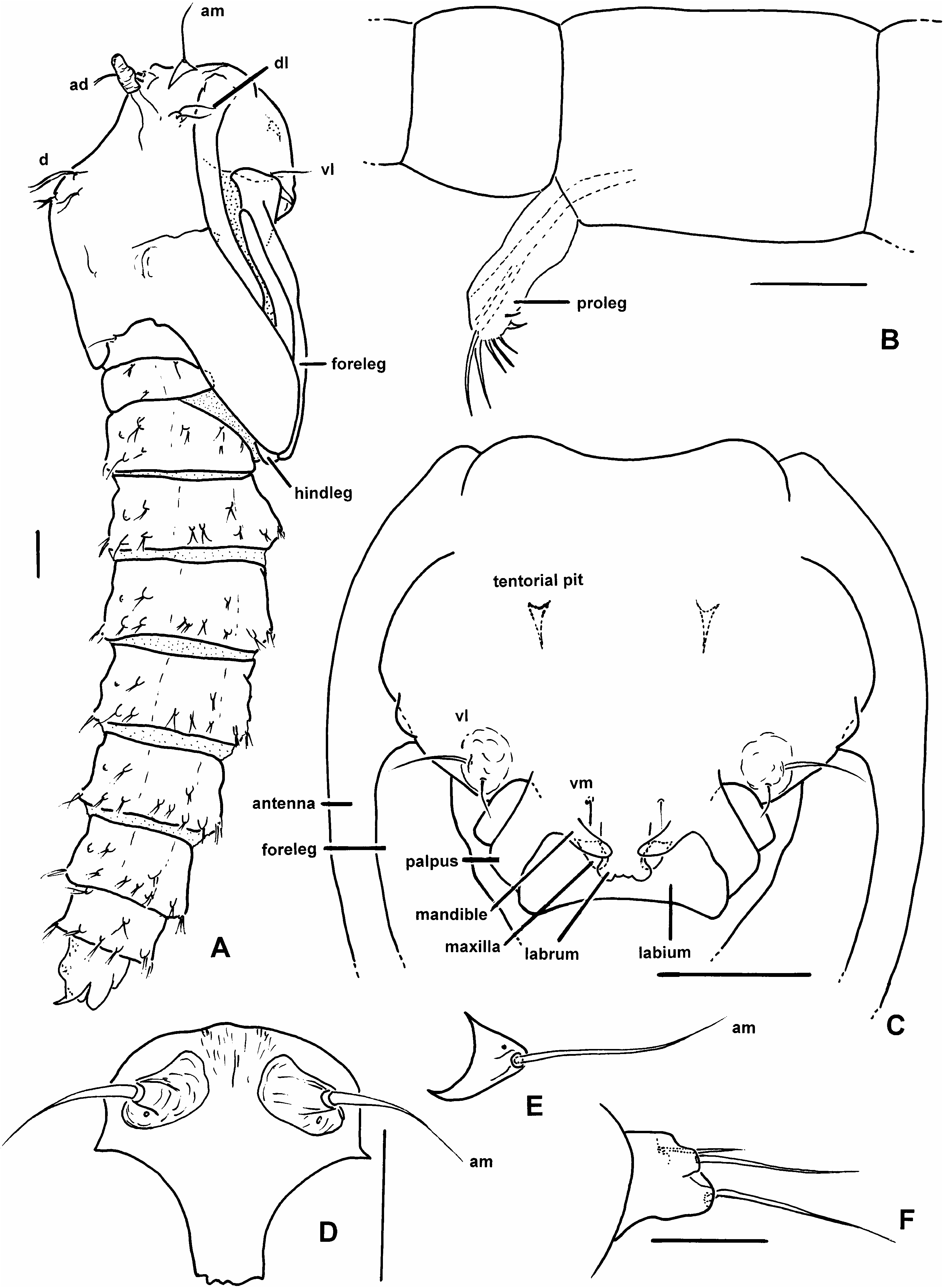
DIAGNOSIS: Male. The only extant Austroconops with flagellomere 12 elongate, 0.6– 0.8 the length of flagellomere 13 ( fig. 1A View Fig ). Female. The only extant Austroconops with simple claws (each with only a very fine basal tooth) ( fig. 1I View Fig ). Egg and larva (all instars). Not distinguishable from those of A. annettae (see generic diagnosis above). Pupa. The only known pupa in the genus (see generic diagnosis above).
DESCRIPTION: Male adult: Descriptive statistics in table 1. Head: Antenna ( fig. 1A View Fig ) with well developed plume. Flagellomere 12 elongate, with subbasal constriction, 0.62– 0.77 length of flagellomere 13 ( fig. 1A View Fig ). Mouthparts moderately long. Palpus ( fig. 1C View Fig ) with 4 segments, segment 3 ovoid in lateral view, with capitate sensilla scattered on surface or in shallow pits. Thorax: Scutellum angular in dorsal view. Wing: Costa extending to or just beyond apex of R 3. Legs: Legs lacking armature. Bristles on midleg tibia, first tarsomere of moderate length ( fig. 1M View Fig ). Midleg tibia without apical spur. Hindleg first tarsomere without thick basal spine or stout setae. Claws simple, each claw apically bifid. Genitalia: In life, rotated about 90°. Apicolateral process absent. Gonocoxite short. Gonostylus mostly straight, tapering to curved apex, apical spine absent. Parameres fused medially. Aedeagus short, setose lobe with ventral plate. Female adult: Descriptive statistics in table 2. Head: Ommatidia narrowly separated dorsomedially. Flagellomeres gradually increasing in length from flagellomere 2 to 13. Mouthparts moderately elongate, mandible ( fig. 1G View Fig ) broad, with fine teeth, most directed laterally or ventrolaterally, laciniae with well developed, fine retrorse teeth. Palpus ( fig. 1E View Fig ) with 4 segments, segment 3 well developed, swollen in lateral view, with capitate sensilla scattered on surface or in shallow pits. Thorax: Scutellum angular in dorsal view. Wing: Costa extending to or just beyond apex of R 3. Legs: Femora, tibiae slender. Legs lacking armature. Midleg tibia without apical spur. Hindleg first tarsomere without thick basal spine or stout setae. Foreleg, midleg, hindleg claws ( fig. 1I View Fig ) slender, evenly curved, each with fine inner setalike tooth. Genitalia: Cercus short. Egg: Descriptive statistics in table 3. Firstinstar larva: Head capsule length statistics in table 4. Total body length 0.76–1.26 mm (n = 6). Secondinstar larva: Head capsule length statistics in table 4. Total body length 1.44–2.13 mm (n = 2). Thirdinstar larva: Head capsule length statistics in table 4. Total body length 2.60–3.72 mm (n = 9). Fourthinstar larva: Head capsule length statistics in table 4. Total body length 3.78– 4.84 mm (n = 3). Pupa: Total length 2.0 mm. General coloration of exuviae, including respiratory organs, light brown. Integument smooth with some fine rounded spicules on dorsal surface of anal segment. Initially, live individuals with reddish hemolymph, after 24 hours with reddish core of tissue, with subcutaneous bluishgreen patches in thorax, abdomen (further developmental details given below). Length of cephalothorax/length of abdomen posterior to cephalothorax = 0.70. Dorsal margin of thorax angular in lateral view ( fig. 4A View Fig ). Operculum ( fig. 4D View Fig ) broad dorsally, narrow ventrally, with elongate anteromedial seta ( fig. 4E View Fig ) directed anteriorly, on well developed tubercle; with single pore; tubercle situated dorsally on operculum. Labral, mandibular, maxillary, labial sheaths ( fig. 4C View Fig ) well developed, palpal sheath short, not extending beyond apex of labial sheath. Anterodorsal setae ( figs. 4A View Fig , 5A View Fig ), two, both elongate, well developed, lying against dorsomedial surface of respiratory organ at about its midlength, with pore on dorsal surface of tubercle. Dorsolateral setae ( fig. 4F View Fig ), three; two elongate, with posterior one slightly thicker, on well developed tubercle; third seta short, arising from near base of tubercle. Single, small ventromedial seta ( fig. 4C View Fig ). Two ventrolateral setae ( fig. 4C View Fig ), lateral one longer, thicker. Metathorax ( fig. 6A View Fig ) with two setae, one pore, lateral seta bifurcate, elongate, medial seta simple, short. Antennal sheath ( fig. 5F View Fig ) apex anterior to apex of portion of midleg lying medial to it. Apex of all legs ( fig. 5F View Fig ) terminating near apex of wing sheath. Wing sheath ( fig. 5F View Fig ) rounded apically, without marginal tubercle. Only apex of hindleg ( fig. 4A View Fig ) visible under lateral margin of wing sheath. Respiratory organ ( fig. 5B, C View Fig ), on well developed pedicel, length = 99 µm, laterally compressed, with anterior and slight posterior bulge in lateral view, with 18 pores abutting, arranged in single, moreorless longitudinal (somewhat anterolaterally to posteromedially) row at apex. Dorsomedial ( fig. 5A View Fig ) a minute seta on anterior margin of scutum. Four dorsal setae ( fig. 5A View Fig ); setae i, ii, iv well developed, bifurcate; sensillum vi well developed, simple seta. Metathorax ( fig. 6A View Fig ) nearly completely divided medially, with bilobed medial protuberance from scutum protruding nearly to posterior margin of metathorax. Abdominal segments 2–8 ( fig. 4A View Fig ) with all setae bifurcate, with a few divided further ( fig. 5D, E View Fig ); sensilla separate from one another (none on common tubercle). Following sensilla present on segment 4 ( fig. 5G View Fig ), all setae on short, well developed tubercles: 2 dasm, i a pore, ii a seta; 4 dpm, i, ii, iv setae, iii a pore; lasm, 3 lpm, 3 vn all setae; vasm a pore. Segment 9 ( fig. 6B View Fig ) with well developed, posterolaterally directed apicolateral process with very apex slightly bent dorsally, bearing single pore near base; genital sac moderately elongate, slightly wider than rest of segment, situated ventrally.
DISTRIBUTION AND HABITAT
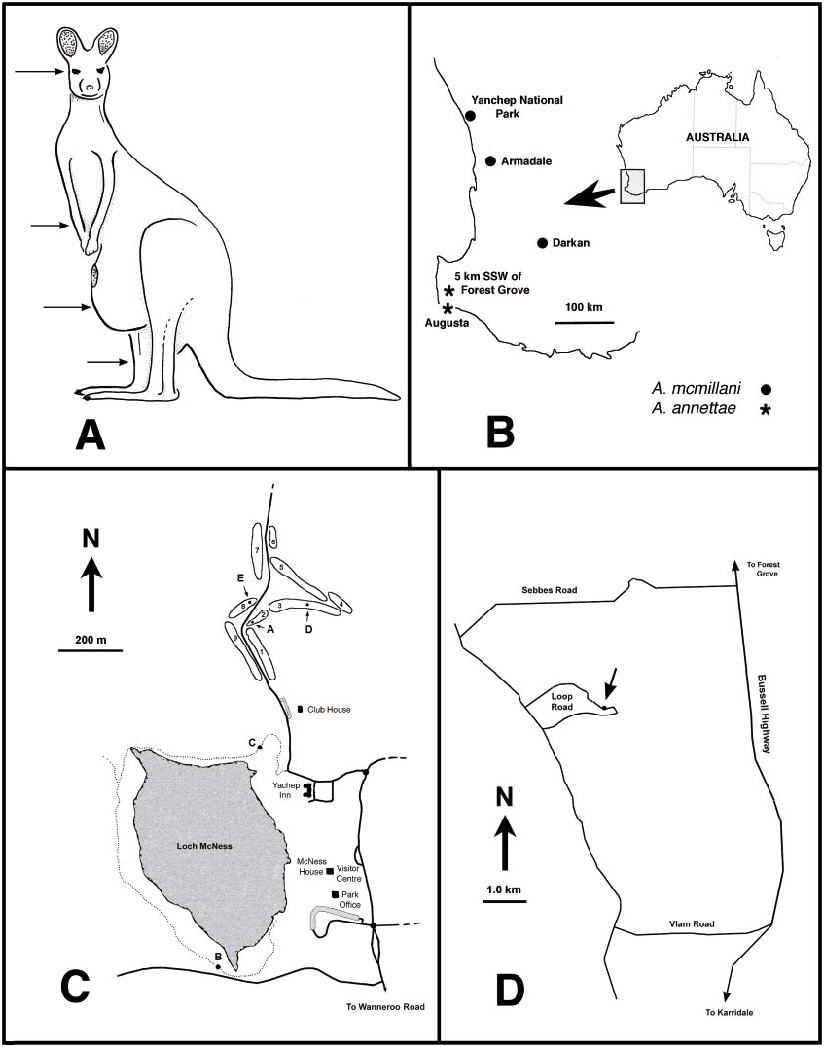
Austroconops mcmillani is known from three sites in southwestern Western Australia ( fig. 22B View Fig ). A fourth locality from ‘‘National Park, Perth’’ cannot be specifically located (see Taxonomic Discussion below).
Adult A. mcmillani were collected at five sites in Yanchep National Park during 2001– 2002 ( fig. 22C View Fig : sites A–E). On November 20–21, 2001 adults were extremely abundant at fairway 2 in the golf course ( fig. 22C View Fig ; site A). Biting females were very common along the southern margin of the fairway ( fig. 21A View Fig ) and at times were attacking humans at the rate of more than 50 per minute! Further observations on biting are given below. Swarming males were collected directly southeast of the teeoff area at the southwestern end of the fairway, which was the driest area on the fairway. The southeastern margin of the fairway had some very wet soil about halfway down its length, and the northeastern end included a ditch with open water. The vegetation included the following trees at the western drier end: Agonis flexuosa (Weeping Peppermint; native to Southwest of W.A., but introduced into the Park), Allocasuarina fraseriana (Common Sheoak) , Banksia attenuata (Yellow Candle Banksia ), Banksia grandis (Bull Banksia ), and Banksia menziesii (Firewood Banksia ). The shrubs and sedges were the same as listed for the eastern (wet) end as given below, but were sparse. Trees at the wet eastern end of the fairway were Banksia littoralis (Swamp Banksia ); shrubs were Acacia saligna (Orange Wattle) , Spyridium globulosum (Basket Bush) , Templetonia retusa (Cockies Tongues) , Xanthorrhoea preissii (Balga or Grass Tree) and sedges were Lepidosperma longitudinale (Pithy Swordsedge), Lepidosperma striatum
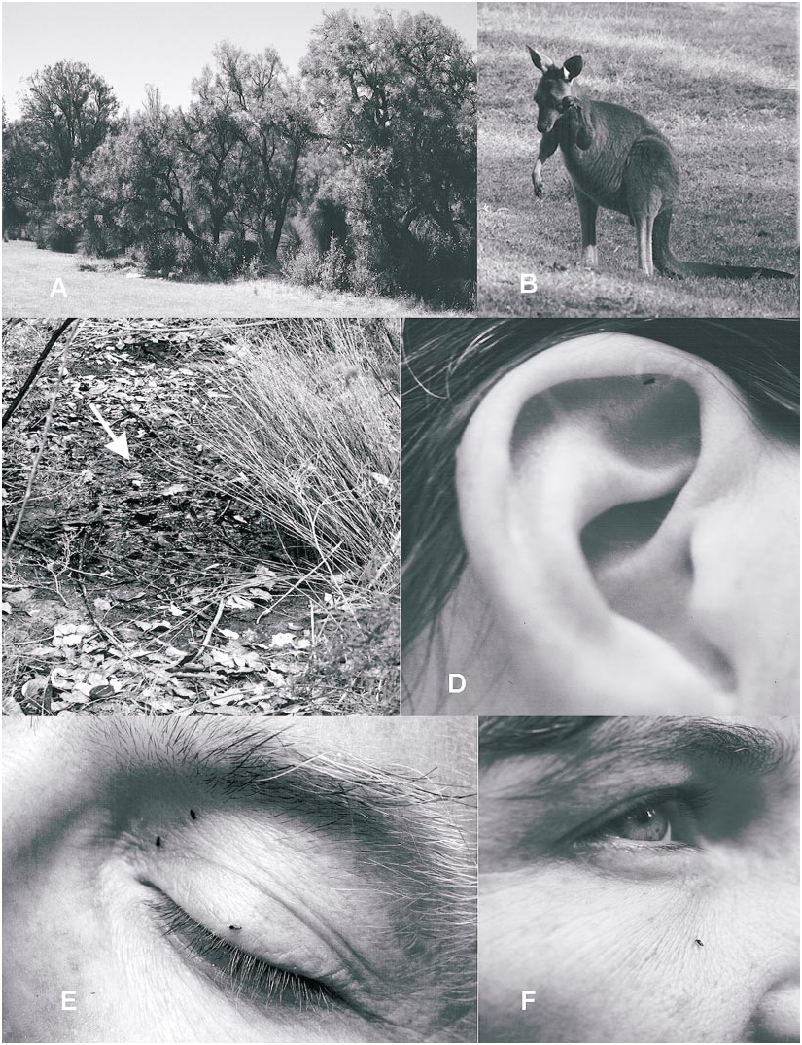
grass. D. A. mcmillani attacking human ear at Yanchep National Park. E. A. mcmillani attacking human eyelid at Yanchep National Park. F. A. mcmillani attacking human cheek at Yanchep National Park.
TABLE 1 Measurements and Ratios of Male Austroconops Wing measurements in mm.
(no specific common name; a swordsedge), and a herb, Centella asiatica (Indian Pennywort) . Samples of mud and detritus from the ditch at the east end of fairway and a few wet soil samples from about threefourths down the fairway failed to produce immatures.
Austroconops mcmillani adults were also observed at the southern margin of Loch McNess ( fig. 22C View Fig ; site B), about 30 meters west of where a small stream enters the lake. The adult population was significantly small er than at site A, and on November 18– 20 females attacked, at best, at the rate of about 1–2/minute. One male was swept from the surrounding vegetation. This wooded area had a narrow innundation zone with very wet soil and mud and some small pools that partially filled after a short rain. The predominant tree at this specific site was Melaleuca rhaphiophylla (Swamp Paperbark) with shrubs of Acacia saligna (Orange Wattle) , sedges Carex fascicularis (Tassel Sedge) , Lepidosperma effusum (Spreading Sword sedge), Schoenoplectus validus (Lake Clubrush) , and some Typha orientalis and a thick mat of an introduced herbaceous weed Pennisetum clandestinum (Kikuyu Grass) . Mud and wet fallen leaves sampled from this site produced no immatures after floating debris and substrate with sugar or sorting by hand.
Two females of A. mcmillani were collected on November 20 at the northeast corner of Loch McNess ( fig. 22C View Fig ; site C). This area was a very wet, but more open, innundation zone.
On November 20 from about 10:50 AM to 1:15 PM the first author and his wife walked completely around Loch McNess ( fig. 22C View Fig ), stopping 14 times at moreorless even intervals around the lake for 3 minutes at a time, breathed deeply (to produce maximum CO 2), and waited for attacking females. The only spots where females were found were at sites B and C ( fig. 22C View Fig ), suggesting that the species is indeed restricted to some specific localities in the park. Females attacked within 5 minutes at site B when arriving at 2:55 PM
TABLE 2 Measurements and Ratios of Female Austroconops Wing values in mm, spermatheca (largest) in µm.
TABLE 3 Measurements and Ratios of Eggs of Austroconops Values given in µm.
(November 19, 2001) and within seconds upon arrival at site A at 10 AM (November 20, 2001), further indicating the presence of quite localized populations of A. mcmillani .
All sites shared the presence of very wet soils, which corresponds well with the behavior of the reared larvae which actively burrowed through wet mud/detritus.
Additional biting females were collected by Len Zamudio at fairway 3 on January 3 and 9, 2002 by a small creek which traverses the fairway about halfway down its length ( fig. 22C View Fig ; site D) and at fairway 8 on January 9 and 31, 2002 ( fig. 22C View Fig ; site E).
The paratype specimens of A. mcmillani collected at Yanchep N.P. were collected on December 23, 1954 by B. McMillan ‘‘at the opening of a small cave to the right of the track leading north from the golf course’’ (A. L. Dyce, personal commun.) and this would represent another place in the park where A. mcmillani are known. However, the total park area has been progressively drying over the past number of years and it is unknown what impact this might have on the distribution of A. mcmillani in the park since 1954.
EGG LAYING, LARVAL AND PUPAL BEHAVIOR AND DEVELOPMENT
Eggs were laid by female A. mcmillani in a scattered pattern on the surface of wet mud in a vial or on the sides of the vial just above the mud where moisture had condensed on the vial wall. Three eggs of A. mcmillani that were laid on the sides of the vial were at least somewhat dried after they were laid and had partially collapsed but when immersed in water and rehydrated, they subsequently hatched. The sequence of egg laying, hatching, and larval development is given in table 5.
Firstinstar larvae burrowed very actively through moderately packed, quite wet mud/ detritus with a serpentine motion. They used different means of propulsion depending on the nature of the substrate and whether they had located food. When larvae were in water, but with substrate to crawl upon, they used their anterior proleg in an anteroposterior motion, dragging the rest of the body along to produce a slightly jerky anterior movement. Larvae that were in very loose detritus and that wanted to move through the water from one clump of detritus to another used their posterior proleg as an anchor, extending
TABLE 4 Head Capsule Lengths of Larvae of Austroconops Values given in µm.
the head and length of the body through water to connect with another piece of detritus. In thicker substrate, at least some movement was facilitated by serpentine movements of the anterior half of the body with the abdomen being dragged behind and with the posterior proleg hooks withdrawn into the anus. In dense substrate (such as thick mud/detritus or agar), the head capsule would additionally be extended anteriorly, bent somewhat down to take hold of the substrate and then bent farther anteroventrally, helping to bring the body forward; these larvae also withdrew their anterior proleg into the body in the crease between the cervix and remaining prothorax. The proleg was withdrawn much like the inverting of a shirt sleeve, with the terminal hooks inverting first into the interior of the extended leg and being withdrawn toward the body and the rest of the proleg following suit. This inversion is almost certainly the result of muscles that insert at the apex of the proleg ( fig. 4B View Fig ). Extension of the proleg was the reverse of this sequence. Larvae removed from substrate and placed in a drop of water on a slide never withdrew the anterior proleg as they actively sought a substrate to latch on to, and it is clear that this structure is important to their locomotion.
As larvae were moving through substrate, their head capsules would move rapidly in a flicking motion left and right and dorsoventrally, apparently testing for potential food. The larvae would also periodically stop, at times anchoring themselves with their posterior proleg and poke their heads rapidly here and there among the detritus, again in an apparent search pattern. The larvae rested regularly and most often used their extended posterior proleg hooks as an anchor on the substrate. Such resting larvae were generally in a moreorless elongate position (with some curves or bends in the body), but occasionally they would assume a tight Ushaped position, with the posterior proleg hooked into detritus.
When firstinstar larvae were removed from very wet mud/detritus to water, they immediately became very active, writhing and attempting to find a substrate upon which to take hold. Upon doing so, they immediately burrowed into the substrate and clearly preferred more solid clumps of detritus than fine, looser particles to burrow into. Larvae in detritus that were harassed with a probe quickly escaped with rapid use of the anterior prolegs and, in thick substrate, some undulations of their bodies.
Five firstinstar larvae of A. mcmillani were observed feeding on E. coli , and when doing so they had their posterior proleg firmly hooked into the substrate and worked over acceptable substrates with their mouthparts. The head capsule moved back and forth, appressed to the agar, and the mouthparts moved rapidly, with the labrum contracting and apparently moving food into the mouth cavity. The pharyngeal complex periodically moved food into the gut. The gut contents appeared white in these individuals (same color as the E. coli ). When feeding, the antennae actively moved independently of one another. After feeding on E. coli for 3 days, all five larvae died.
When drops of the fecal infusion, nematodes, and rotifers were introduced to the larvae in the mud/detritus substrate, further observations were made of feeding. Larvae were obviously attracted by the drops of fecal infusion and were often very concentrat ed directly underneath these small pools of feces. Occasionally, larvae would be seen lashing at a passing Paramecium or a slower rotifer. A few secondinstars were seen to successfully attack and eat passing rotifers but missed most that were nearby. The strong impression of all larval instars was that they could at best only attack slow prey.
Secondinstar larvae became increasingly fat and slower and by the thirdinstar and especially by the fourthinstar, larvae moved more cumbersomely, with a slow, serpentine motion. Serpentine movements of the body were obviously the most important basis for movement as the larvae worked their way through the mud/detritus substrate. To re
text. Numbers refer to the fairways at the golf course. D. Map of the type locality of Austroconops annettae at 5 km SSW of Forest Grove , Western Australia .
verse direction, a larva either simply reversed its serpentine movement or, by turning its head capsule and anterior portion of the thorax in a posterior direction, doubled back along the length of its body The anal hooks were withdrawn into the anus when a larva moved and were often (but not always) exserted when the larva rested; in some instances the hooks were attached to substrate, but in more sparsely distributed substrate were simply exserted into the water without being anchored to anything. The anterior proleg also appeared to be rarely used by third and fourthinstar larvae and apparently was exserted only when the larva had difficultly finding any substrate ahead. Second, third and fourthinstar larvae always extend ed their anterior proleg in a groping motion when placed in a drop of water on a microscope slide and writhed helplessly, very similar to the more ponderous movements of Dasyhelea larvae. Larvae which were harassed with a probe quickly escaped with rapid serpentine undulations of their bodies through the surrounding substrate. When the substrate was shaken and larvae severely dis
TABLE 5 Data on Rearing of Austroconops mcmillani (2001–2002)
turbed, fourthinstars often assumed a curledup position (this was not attempted with earlier instars). The darting, probing search pattern of the head capsule continued in these stages. Larvae sometimes moved their antennae and, when ingesting food, moved their mandibles synchronously. Some secondinstar and most thirdinstar larvae took on a pinkish hue. A few thirdinstar larvae remained unpigmented and translucent. All fourthinstar larvae were pink or reddish.
The day before the single fourthinstar larva pupated, its thorax was strikingly broad, the respiratory organs of the pharate pupa could be clearly seen, and the eyespots had moved posteriorly in the larval head capsule. The pupa, which completely shed the larval exuviae, was initially very pale, with red hemolymph, and, at 22–25°C, was very sluggish, although capable of very slow circular movements of the abdomen. It was buried in the wet substrate at about a 50–60° angle from the surface, with the tip of one respiratory organ just sticking out of the water; later, the tips of both respiratory organs were exposed. Throughout its development the pupa had the apex of either or both respiratory organs exposed to air. After 24 hours, the pupal cuticle appeared nearly transparent (except for the brown respiratory organs), with an inner core of reddish tissue surround ed by completely clear fluid and a few subcutaneous bluishgreen patches in the thorax and abdomen. It was virtually motionless, and flooding for 16 minutes (so that the respiratory organs were submerged) did not result in any pupal movement, nor did any very slight jarring of the surrounding substrate. At one point, upon turning on a bright microscope light, the pupa briefly moved its abdomen in small circular motions. After 48 hours, the pupa did not look much different from 24 hours earlier. After 72 hours, the interior of the pupa became somewhat darker. The bluegreen patches were also present in the head (this may have been true earlier but the head could not be seen clearly then). The pupa was very lethargic and moved only slightly when bright light was suddenly shone on it. At 84 hours the developing adult eyes became visible. At 96 hours, the developing adult had darkened further, with the bluegreen patches more extensively developed. At 108 hours, the dark adult appeared to be moreorless completely developed and its black head and thoracic cuticle could be clearly seen. Shortly before the adult male emerged, at 120 hours after pupation, the pupa slowly wriggled its way out of its circular burrow and lay on the wet mud surface. The adult emerged during daylight hours (early afternoon) but failed to completely expand its wings (they were entrapped by the wet mud surface); in all respects it appeared normally developed and a hindleg first tarsomere length of 176 µm (males in nature = 178–214 µm, N = 13) indicated that the rearing conditions and food provided to the larva was of sufficient quality to produce a typical adult.
BITING ACTIVITY AND BEHAVIOR
Of those female adults of A. mcmillani biting humans at Yanchep National Park, most bit on the face ( fig. 21D, F View Fig ); less than 15% of individuals fed elsewhere on the body (mostly shoulders, arms, legs). Females on the face clearly favored areas without hair and, of those attacking the face, were primarily close to the eyes and directly on the ears, with fewer on the cheeks and forehead. A few fed among hairs right at the hairline, including the scalp, eyebrows, eyelashes, and the first author’s beard. We are unable to suggest the cues used by females to preferentially attack the face. When bare arms were held up and appressed against the face, the face was still preferred; however, in such a position a few females attacked the armpit (no deodorants used), suggesting that secretions of the skin may be involved as an attractant. Slightly elevated heat from these areas cannot be excluded but this seems unlikely, since females were not attracted to handheld cups of warm or hot water, as are females of some species of Culicoides and Culicidae . It is probable that female A. mcmillani were attracted by CO 2. At the south end of Loch McNess ( fig. 22C View Fig ; site B) when the frequency of biting decreased, the number of incoming females could be increased within 3–5 minutes by doing onthespot exercises or by intentionally breathing deeply and rapidly.
Females flew about the head for a period before landing. Those landing on the hair nearly always quickly flew off the surface to resume hovering. After landing on skin, nearly all females began feeding immediately (very little walking about on the skin surface).
Kangaroos were closely observed during the first sampling period of October 16–20, 2001, before the emergence period of A. mcmillani . They very rarely scratched or rubbed themselves. When the first author and his wife were experiencing very intense biting on the golf course in Yanchep N.P. on November 20–21, it was clear that the kangaroos were also being severely bothered by midges ( figs. 21B View Fig ). Many rubbed their eyes and scratched at their forelegs and the shins of the hindlegs with their foreleg feet ( fig. 22A View Fig ). Female kangaroos carrying well developed joeys (baby kangaroos) also often scratched at the front of their extended pouches. The midges attacked the eyes and areas with a minimum of hair. The first author chased kangaroos with an aerial net during these observations and collected numbers of A. mcmillani females, but it was uncertain if these specimens were attacking the kangaroos, were attracted to the first author, or were merely free flying in the area.
Females assumed a biting posture similar to those of Leptoconops and Culicoides that the first author has examined, with the body virtually parallel to the skin surface but very slightly elevated posteriorly ( fig. 21D, F View Fig ). Of 27 females studied, total time to complete blood feeding ranged from 1:05 to 4:40 minutes:seconds (mean = 2:42), when ambient air temperatures ranged from 22 to 29°C (taken in shade). There was no significant correlation between temperature and biting time, although all the feeding times longer than 3 minutes (N = 8) were at temperatures at or below 25°C. We found the bite of female A. mcmillani to be more painful than those of other ceratopogonids that we have experienced ( Leptoconops , Culicoides ), perhaps because they concentrated on the more tender facial area.
Females fed only during the day when temperatures were above at least 21°C. On November 21 females began feeding at fairway 2 ( fig. 22C View Fig ; site A) at 7:50 AM, when the ambient temperature was 22°C. On November 18, at the south end of Loch McNess ( fig. 22C View Fig ; site B), they ceased attacking by 5:45 PM as the light was fading and the temperature was 24°C. Females in vials became lethargic at about 18–19°C and at about 16– 17°C were nearly torpid. It appeared that stronger winds dampened activity, although females continued to bite in sheltered areas, such as the leeward sides of trees, a behavior typical of many biting ceratopogonids. High temperatures (generally above 24°C) and increased humidity appear to greatly increase biting rates (L. Zamudio, personal commun.). Cloudy weather and even a light rain did not stop females from attacking, although a steady drizzle did stop biting activity on the afternoon of November 18, 2001.
ADULT SEASONALITY
Intense collecting of Ceratopogonidae in Yanchep National Park showed that no A. mcmillani adults were present October 16– 20, 2001. November 5 produced moderate numbers of females, which slowly increased with large numbers biting on November 18. Biting activity varied, depending on environmental conditions, until at least January 31, 2002, indicating a nearly 3month period of female adult biting activity. Populations in the 2002–2003 season were substantially less at Yanchep N.P. (possibly due to a very dry season; L. Zamudio, personal commun.) and biting female adult A. mcmillani were col lected from November 14, 2002 until January 1, 2003. Two female A. mcmillani were sampled from kangaroo feces on January 21, 2003 (L. Zamudio, personal commun.), indicating that the emergence period was still about 3 months long in the 2002–2003 season.
Records from three years show males have been collected Oct. 22–23 and Nov. 1, 1985; Nov. 19–21, 2001; Nov. 20, Nov. 22, Dec. 6, and Dec. 12, 2002.
MALE SWARMING
Male swarms were observed on November 21, 2001 on fairway 2 of the golf course at Yanchep National Park ( fig. 22C View Fig ; site A) from 6:45 to 9:15 AM. At 6:45 the temperature was only 15°C (in shade, warmer in sun) and calm. At 7:50 AM and 22°C a single, small (about 30 cm in diameter), moreorless spherical swarm was seen about 1 meter above the grass and about 6 meters from the margin of short trees at the edge of the fairway. A light wind began blowing. Several similarly situated swarms were seen during the next 40 minutes. The swarms started to increase in size, became more elongate vertically, and moved to within 3 meters of the leeward side of the trees, just south of the teeoff area. No obvious swarm markers were evident, other than being on the leeward side of the trees. By 8:05 AM the bottom of the swarms were 2–3 meters above the ground and larger (about 50 X 100 cm) and withstood some wind, albeit being periodically blown about and then regrouping. Swarming activity ceased in this immediate area at about 8:30 AM but continued a short distance farther east along fairway 2 (where it was a bit cooler) and then began in the first area again at 8:37 AM for at least 30 minutes. One swarm collected at 9:00 AM with a rapidly swept aerial net consisted of 160 males and 10 females (the latter may have been captured because females had begun biting by then). By 9:15 AM swarming had ceased and did not resume for the next 30 minutes, at which time observations were terminated.
A male and a female of A. mcmillani were collected in copula at 8:47 AM during these observations by sweeping from ground level to about 1 meter in height in the nearby vi cinity of the swarms; the pair separated after about 20 seconds while in the net. While still attached the pair stood on the net mesh facing in opposite directions, showing that the male can at least opportunistically rotate his genitalia 180° (they are otherwise generally held at about 90°). Small white sheets (about 1 X 1 m) were placed on the ground under the swarms of males but no mating pairs settled on these. The fact that females captured immediately after they had completed feeding were able to lay fertile eggs proves that mating occurs before feeding.
OTHER OBSERVATIONS
On November 21, 2002 females did not attack the first author on fairway 2 in Yanchep N.P. before 7:50 AM. From 6:15 until 6: 45 AM females were sampled by dropping a net over one or more fresh (wet) kangaroo feces and then disturbing the feces with a stick slipped under the margin of the net that was appressed against the grass. Some females were sampled by kicking at the feces and then immediately sweeping briskly over the area. In virtually every patch, some females were collected, with numbers varying from 0 to 8 individuals (58 collected in 30 minutes). Three attempts at collecting females from dry kangaroo feces failed to produce any specimens. This suggests that the females were imbibing nutrients from the wet feces but further observations are required. That reared larvae obtained nutrients from a fecal infusion in the laboratory might suggest the possibility that the females were laying eggs on or near the kangaroo feces, but most of the feces were in an area with normal soils, and the clearly aquatic larvae of A. mcmillani could almost certainly not survive there.
Of 280 preserved male A. mcmillani collected from swarms in Yanchep National Park on November 21, 2001, one specimen had a phoretic female mite intertwined between its legs. The mite belongs to an undescribed species of Blattisocius Keegan (Ascidae) (E. Lindquist, personal commun.). This mite genus is virtually cosmopolitan, and 11 of the 12 known species are, or are likely to be, predators (the twelfth is an ectoparasite in noctuid moth ears). No obvious internal parasites (e.g., no nematode infestation) were seen among the specimens examined.
Female adults clean their antennae with the foretibial spurs. The wings were cleaned by opening the wings very slightly and hooking one hindleg over the anterior margin of the wing and pushing it alongside and under the abdomen where both hindlegs rubbed their tarsi against each corresponding wing surface (so that the left leg rubbed the dorsal surface of the left wing and the right leg rubbed its underside). After wing cleaning, the wings were sometimes left at an angle but shortly after ‘‘clicked’’ back to the typical overlapping position over the abdomen. The hindleg tarsi were cleaned by rubbing them against each other under the body.
TAXONOMIC DISCUSSION
The adult male and female of A. mcmillani were described in some detail by Borkent et al. (1987). There is some confusion about the exact location of the type locality, recorded on the labels of the type series as ‘‘National Park, Perth, W.A., 21XII1954 ’’. There is not, and has never been, a national park in Perth, although the renowned 4km 2 Kings Park may have been a candidate in the mind of the collector. More likely, the labels may refer to John Forrest National Park, the first national park in Western Australia, located about 22 km northeast of Perth city center. An old ‘‘Caltex’’ roadmap in the possession of the first author, probably from the 1950s or 1960s, indicates John Forrest N.P. merely as ‘‘National Park’’, perhaps confirming that this is actually the type locality. The original collector, B. McMillan, is deceased (A.L. Dyce, personal commun.). The holotype is housed in the ANIC.
Wirth and Lee (1958) recorded the following original material, all females: holotype and 16 paratypes from ‘‘ Perth National Park’ ’, 9 paratypes from Yanchep National Park , 11 nonparatypes from 10 miles SE of Darkan and two nonparatypes from Armadale. We were unable to locate 5 paratypes from ‘‘ Perth National Park’ ’, 1 paratype from Yanchep National Park , and 10 of the specimens from 10 miles SE of Darkan, and these appear to be lost (A.L. Dyce, personal com mun.). The specimens we examined from Armadale and 10 miles SE of Darkan are labeled as paratypes, although they were excluded from the type series in the original publication. Furthermore, a specimen from ‘‘ Perth National Park’ ’ (ANIC) was not labeled as a paratype although it almost certainly is one. The slide label is written in W.W. Wirth’s handwriting and the specimen is mounted in his typical fashion; we have added a paratype label to indicate this.
The female adults from Yanchep N.P. were larger than most of the specimens from farther south. The following are the ranges and means of wing lengths for the limited specimens available: Yanchep N.P., 0.82–0.93 mm, 0.89 (N = 16); ‘‘Perth N.P.’’, 0.80–0.83 mm, 0.81 (N = 4); Armadale, 0.71–0.79 (N = 2); Darkan, 0.82 (N = 1). This may indicate the possibility of clinal variation, but further specimens and study are required to determine this.
The pupal dorsal setae are here labeled as i, ii, and iv. Based on their position on the thorax, setae i and iv are likely homologous to those named as such in other Ceratopogoninae . However, seta ii could equally be homologous to seta iii of the Ceratopogoninae .
SPECIMENS EXAMINED
Yanchep National Park , Nov. 18–21, 2001: 442 males, 201 females; Yanchep National Park from females captured Nov. 20, 2001: 10 eggshells, 2 eggs, 1 eggshell with firstinstar stuck during emergence, 10 firstinstar larvae, 2 secondinstar larvae, 8 thirdinstar larvae, 3 fourthinstar larvae, 1 pupal exuviae and associated male ( CNCI) ; Yanchep National Park , golf course, Dec. 11, 2001 – Jan. 31, 2002: 32 females ; Yanchep National Park , Oct. 22–Nov. 1, 1985: 3 males ( ANIC, CNCI) , 5 females ( ANIC, CNCI, WAMP) ; Yanchep National Park , Dec. 23, 1954: 4 female paratypes ( BMNH, BPBM, USNM, WAMP) ; Yanchep National Park , golf course, Nov. 14, 2002 – Jan. 1, 2003: 55 males, 1833 females ; Armadale , Jan. 4, 1936: 2 females (labeled as paratypes) ( ANIC, USNM) ; Darkan , Jan. 29, 1953: 1 female (labeled as paratype) ( USNM) ; Perth National Park , Dec. 21, 1954: 3 female para types, 1 female not labeled but likely a paratype (new paratype label added) (3, USNM; 1, ANIC) .
In addition to the above material, more eggs, eggshells, and specimens of the different larval instars were studied but were either left to develop or were subsequently lost. Therefore, the numbers in tables 3 and 4 recording numbers of specimens does not match that listed above.
DERIVATION OF SPECIFIC EPITHET
The species was named by Wirth and Lee (1958) after the collector of the type series, B. McMillan.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
InfraClass |
Lower |
|
Order |
|
|
Family |
|
|
Genus |
Austroconops mcmillani Wirth and Lee
| BORKENT, ART & CRAIG, DOUGLAS A. 2004 |
Austroconops mcmillani: Borkent, Wirth and Dyce, 1987
| : Borkent, Wirth, and Dyce 1987 |
Austroconops mcmillani
| Wirth and Lee 1958: 337 |