Gomphosphenia patrickiana Cantonati, Lange-Bert., Kociolek & A.A.Saber, 2021
|
publication ID |
https://doi.org/ 10.5252/cryptogamie-algologie2021v42a13 |
|
DOI |
https://doi.org/10.5281/zenodo.7819281 |
|
persistent identifier |
https://treatment.plazi.org/id/FA5FD005-FFFA-DB10-FEB5-66E3FAB7F8E5 |
|
treatment provided by |
Felipe |
|
scientific name |
Gomphosphenia patrickiana Cantonati, Lange-Bert., Kociolek & A.A.Saber |
| status |
sp. nov. |
Gomphosphenia patrickiana Cantonati, Lange-Bert., Kociolek & A.A.Saber , sp. nov.
( Figs 4-6 View FIG View FIG View FIG )
TYPE MATERIAL. — Unites States. Puerto Rico, Cupeyes Stream, National Forest , south-western part of the Island of Puerto Rico, lithology: serpentinites (metamorphic rocks), (18°06’48.65”N, 66°59’12.30”W, 166 m a.s.l.), 3.II.2016 (holo-, ANSP, slide NEON00303 b, partly shown here in Fig. 4 View FIG ; GoogleMaps iso-, BM, slide BM 81900; iso-: TR, slide cLIM004 DIAT 3904; NEON Biorepository, Arizona State University’s Natural History Collection in Tempe, AZ, slide NEON 00303 a; diatom collection of the Botany Department, Faculty of Science, Ain Shams University, Egypt, slide PBA– DIAT 2001).
REGISTRATION. — http://phycobank.org/102720
ETYMOLOGY. — The specific epithet “ patrickiana ” is named in honor of the United States phycologist and limnologist specializing in diatoms and hydrobiology, Ruth M. Patrick (1907-2013). She developed innovative ways to assess the quality of freshwater ecosystems, in particular using diatoms, authored>200 scientific papers, and established numerous research facilities, in particular the Phycology Section at the Academy of Natural Sciences of Philadelphia (ANSP, part of Drexel University since 2011). We consider this last achievement notable in the international museological context, as a bright example of a part of the research division (ANSP’s Patrick Center for Environmental Research) of a science museum that obtains most of its funding from tenders, consulting, and ecological assessment projects, many carried out at a nation-wide scale.
MORPHOLOGY
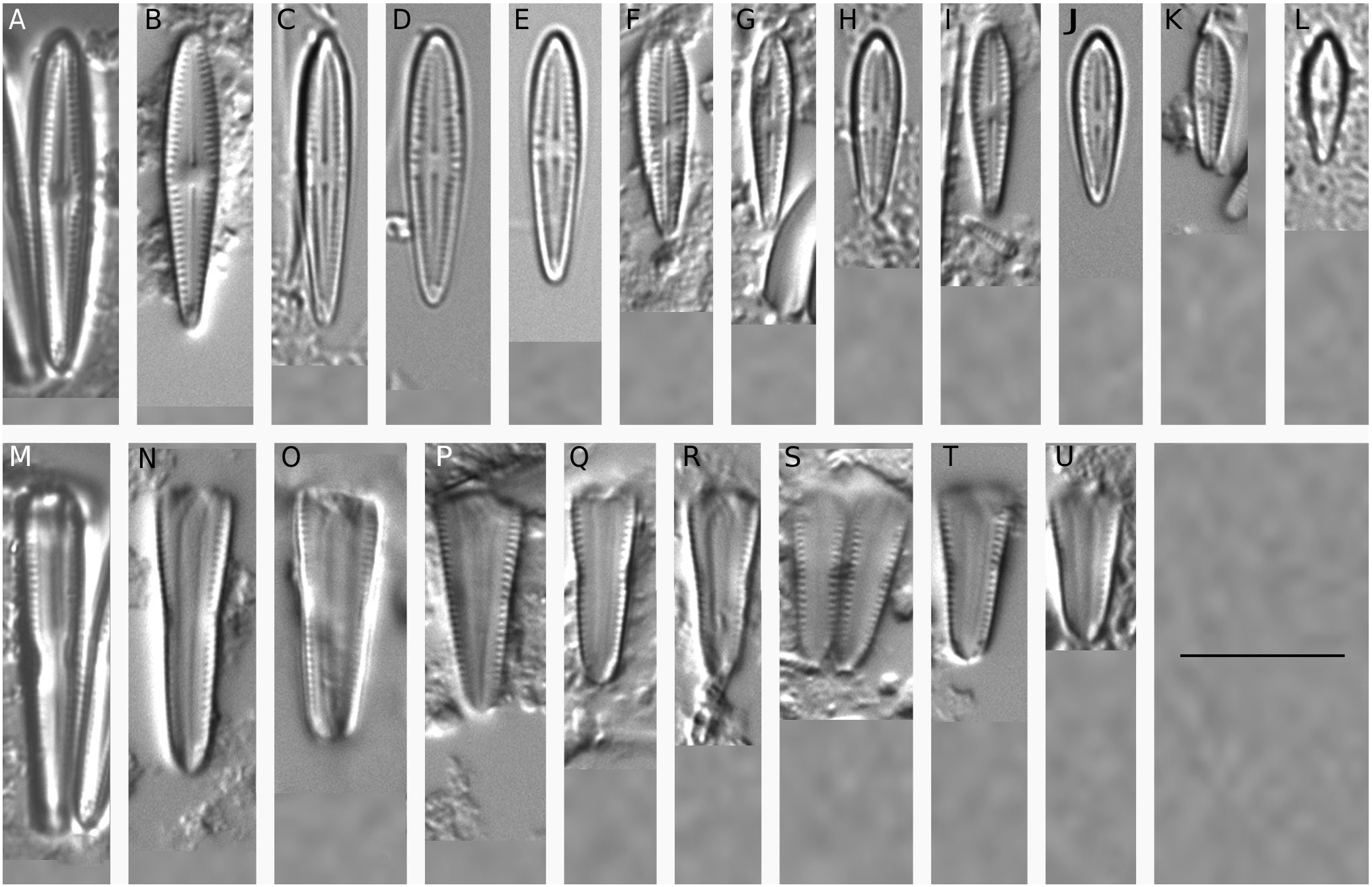
Light microscopy ( Fig. 4 View FIG M-U)
Frustules wedge-shaped in girdle view ( Fig.4M - U View FIG ).Valves linearclavate in larger specimens to clavate in smaller ones, slightly broader at the central area in the larger specimens, the headpoles rounded whilst the footpoles are narrowly rounded ( Fig. 4 View FIG A-L), 8.0-17.5 µm long, 2.5-4.0 µm wide, L/W ratio: 3.4-4.8. Axial area lanceolate to moderately narrower in the smaller specimens, in general narrow at the poles and gradually widening towards the central part. Central area ± rhombic, mostly with 2-4 shortened striae, and in some specimens transversely expanded to the valve margin on one side forming a unilateral fascia ( Fig. 4C View FIG ). The raphe straight, filiform with distinct proximal endings. Striae radiate throughout the valve, becoming almost parallel near the head- and footpole, 23-27 in 10 µm.
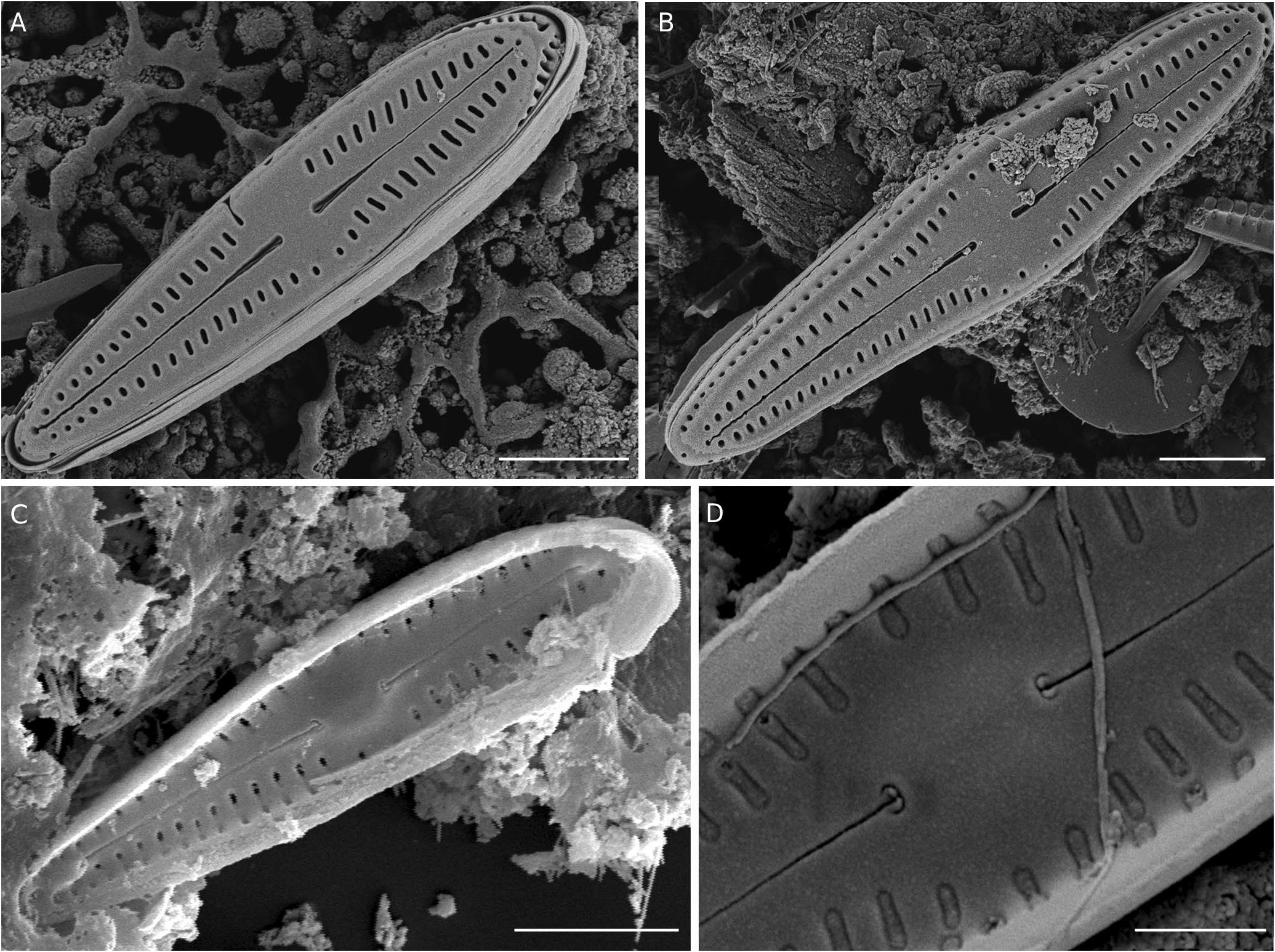
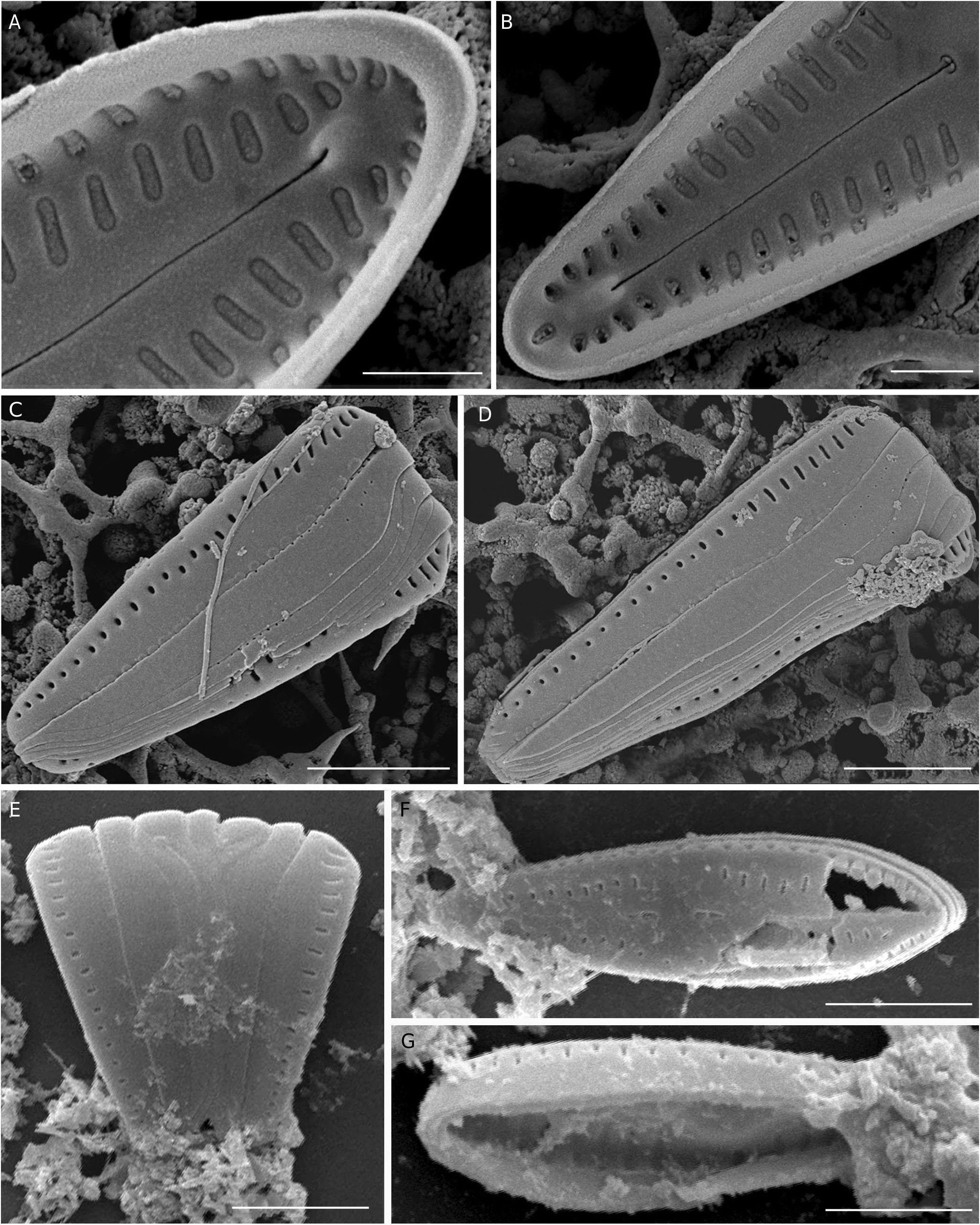
Scanning electron microscopy ( Figs 5 View FIG ; 6 View FIG )
The valve face is flat, without stigma. Striae on the valve exterior composed of single radiate slit-like areolae ‘foramina’, becoming almost round poroids near the poles, particularly the footpole ( Fig. 5A, B View FIG ). Central area in some specimens with a broad unilateral fascia ( Fig. 5A View FIG ), and slightly broader in the larger specimens ( Fig. 5B View FIG ). Raphe straight, filiform with distinctly dilated proximal raphe endings, and distal raphe fissures terminating on the valve face, not extending into the valve mantle, with teardrop-shaped pores ( Fig. 5A, B View FIG ). The valve mantle bears a single row of areolae which are slit-like at the upper valve half and the headpoles but rounded in shape near the mid-valve and towards the footpole ( Fig. 6C, D View FIG ). Internally, septa and pseudosepta at both poles are absent ( Fig. 5C View FIG ; 6F, G View FIG ). Internal foramina have almost the same size as the external areolae, and are occluded by hymenes ( Fig. 5C, D View FIG ). Central nodule is slightly raised ( Fig. 5D View FIG ). Internal proximal raphe endings are T-shaped ( Fig. 5C, D View FIG ), while the internal terminal raphe fissures terminate in relatively large helictoglossae ( Fig. 6A, B View FIG ). In girdle view, frustules typically wedge-shaped, with cingulum composed of seven open bands, each bearing one row of small round pores. Apical pore fields absent ( Fig. 6C, D, E View FIG ).
ECOLOGY AND CO- OCCURRING DIATOM SPECIES
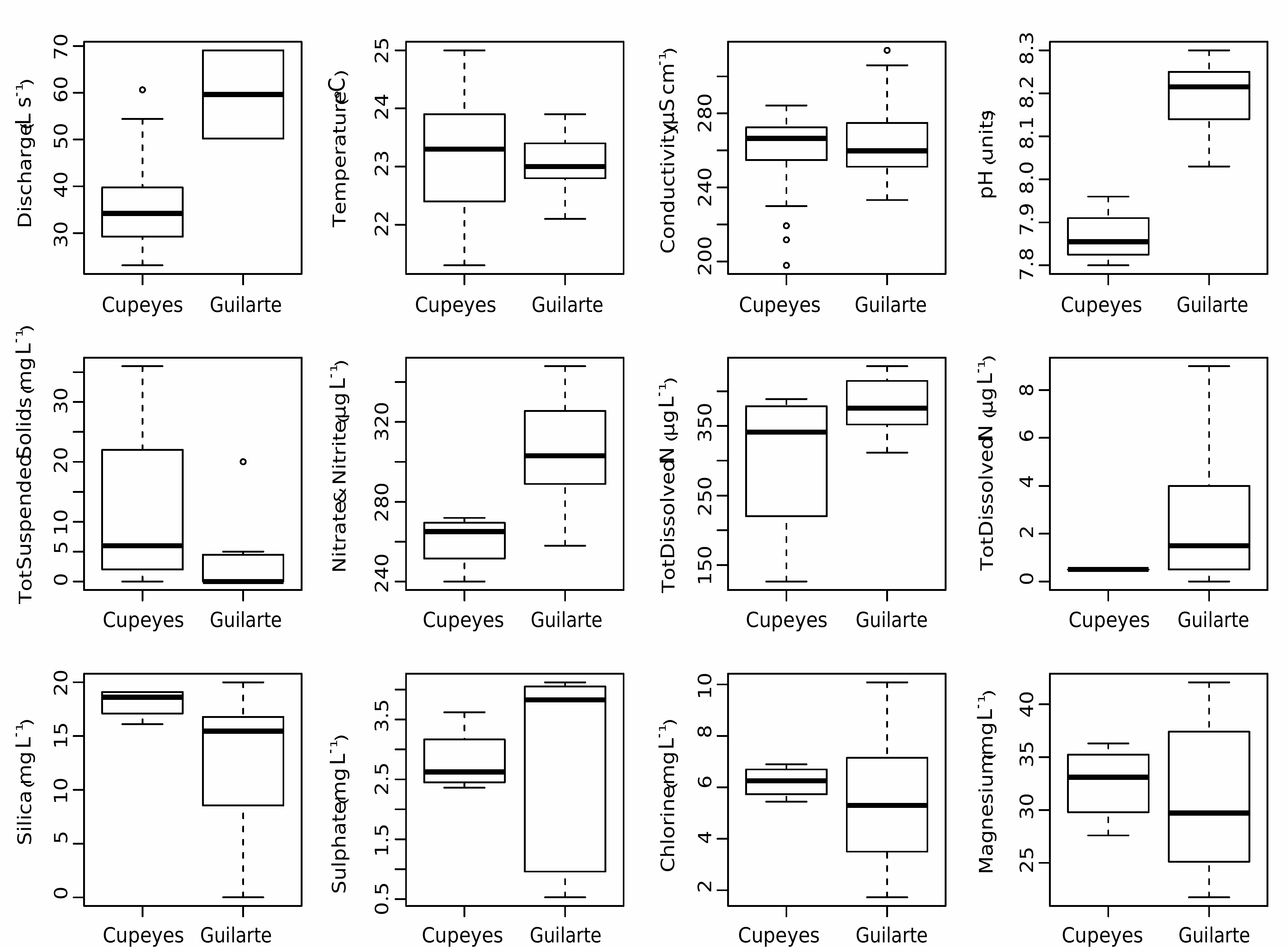
So far, G. patrickiana has been observed only as a Neotropical epilithic species in the two rivers Rio Cupeyes and Rio Guilarte, located in southwest Puerto Rico. It was reported in 27 out of the 37 samples investigated from Rio Cupeyes, and in 21 out of the 45 samples from Rio Guilarte. Maximum relative abundance was distinctly higher in Rio Cupeyes (43%) compared to Rio Guilarte (3%). The Rio Cupeyes is, in general, characterized by a water quality much better than that of the agricultureimpacted Rio Guilarte stream, in terms of average TDN and TDP values ( Fig. 1 View FIG ).
Only epilithon samples were available for this study but they were collected from different habitats and stream reaches (within the same station). Table 1 View TABLE shows the distribution of the species (using per cent relative abundance) with respect to stream and microhabitat, showing that it was clearly more abundant and frequent in the Rio Cupeyes (Kruskal-Wallis chi-squared “streams” = 14.37, d.f. = 1, p = 0.0001). Similar results were obtained for microhabitats (Kruskal-Wallis chisquared “habitats” = 11.01, d.f. = 2, p = 0.004) and seasons (Kruskal-Wallis chi-squared “seasons” = 10.92, d.f. = 2, p = 0.004). However, a three-way ANOVA showed that the interaction among these three factors is significant (factor = river × season, d.f. = 2, F = 8.20, p = 0.001; factor = season x habitat, d.f. = 4, F = 3.95, p = 0.006).
The predominant diatom species (relative abundance> 5%) of the most common genera at the type locality (Rio Cupeyes) during the whole period of study were: Gogorevia constricta (Torka) Kulikovskiy & Kociolek , Achnanthidium jackii Rabenhorst , Adlafia neoniana Cantonati , Denticula occidentalis Østrup , Gomphonema neotropicum N.Abarca & D.Mora , Nitzschia paleacea (Grunow) Grunow , Sellaphora saugerresii (Desmazières) C.E. Wetzel & D.G. Mann , and Ulnaria lanceolata (Kützing) Compère. Predominant species (rel. ab.> 5%) at the Rio Guilarte were: Achnanthidium eutrophilum (Lange-Bert.) Lange- Bert., Cocconeis placentula var. euglypta (Ehrenberg) Grunow , C. placentula var. lineata (Ehrenberg) Van Heurck , Craticula subminuscula (Manguin) C.E. Wetzel & Ector , and Cymbella turgidula Grunow , Gomphonema kobayasii Kociolek & J.C. Kingston , Halamphora veneta (Kützing) Levkov , Nitzschia cf. palea (Kützing) W.Smith , Sellaphora nigri (De Notaris) Wetzel & Ector , Ulnaria monodii (Guermeur) Cantonati & Lange- Bert., and U. ramesii (Héribaud) T. Ohtsuka.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |