Trioza incrustata Percy
|
publication ID |
https://doi.org/ 10.1080/00222933.2015.1104394 |
|
DOI |
https://doi.org/10.5281/zenodo.4329231 |
|
persistent identifier |
https://treatment.plazi.org/id/FB2487A1-4359-FFF1-FE30-FE56676B4D77 |
|
treatment provided by |
Carolina |
|
scientific name |
Trioza incrustata Percy |
| status |
sp. nov. |
Trioza incrustata Percy , sp. nov.
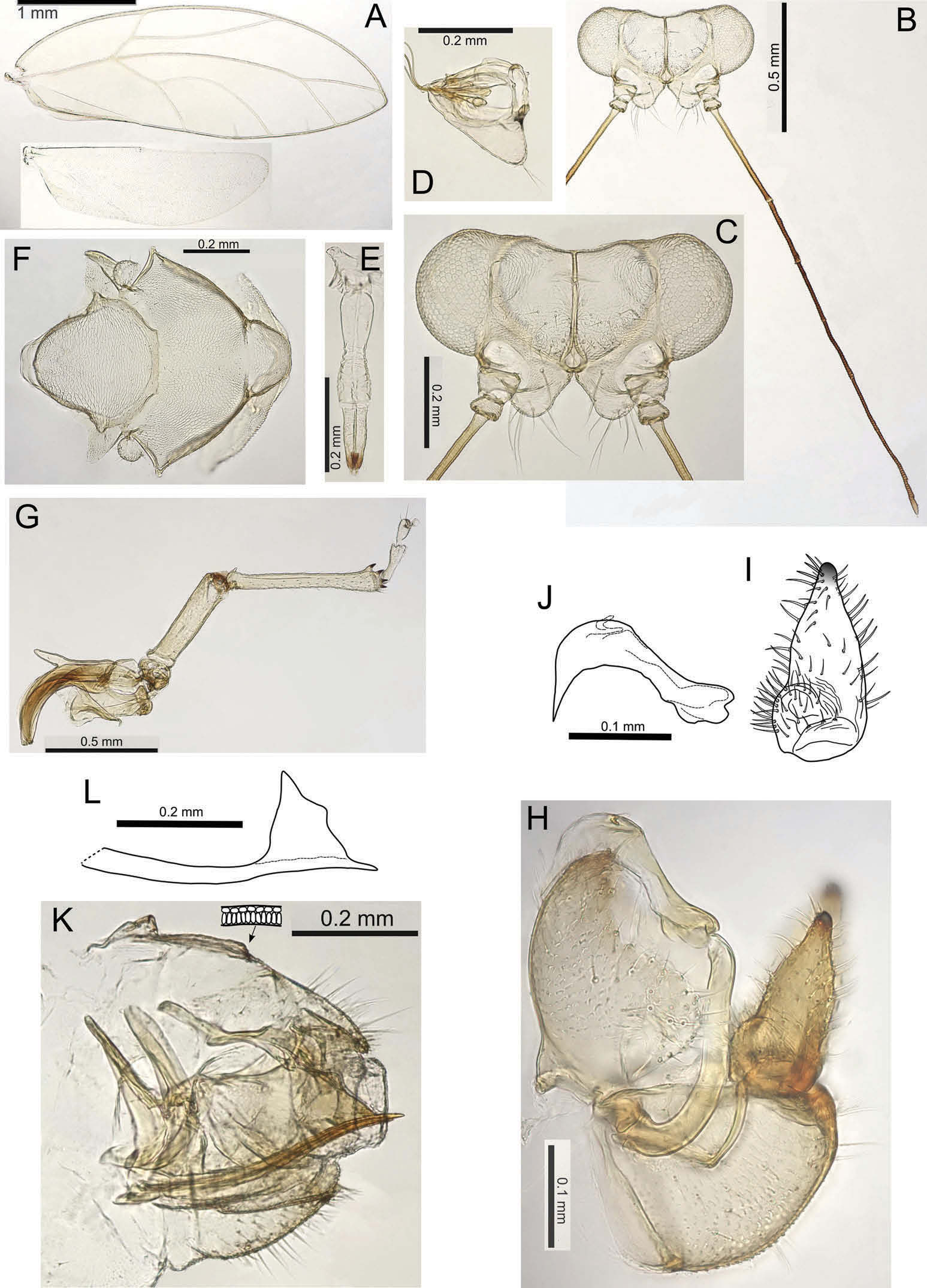
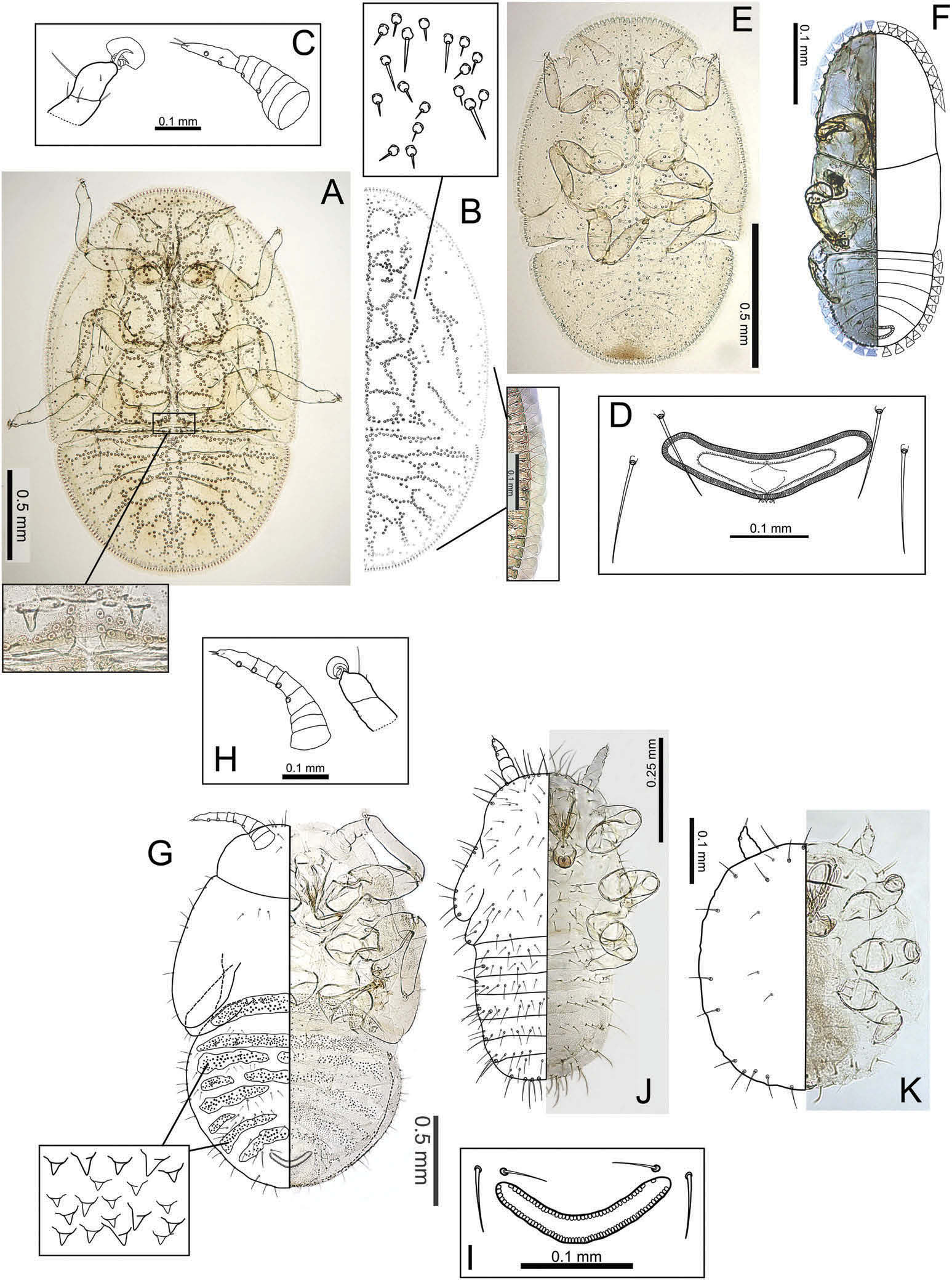
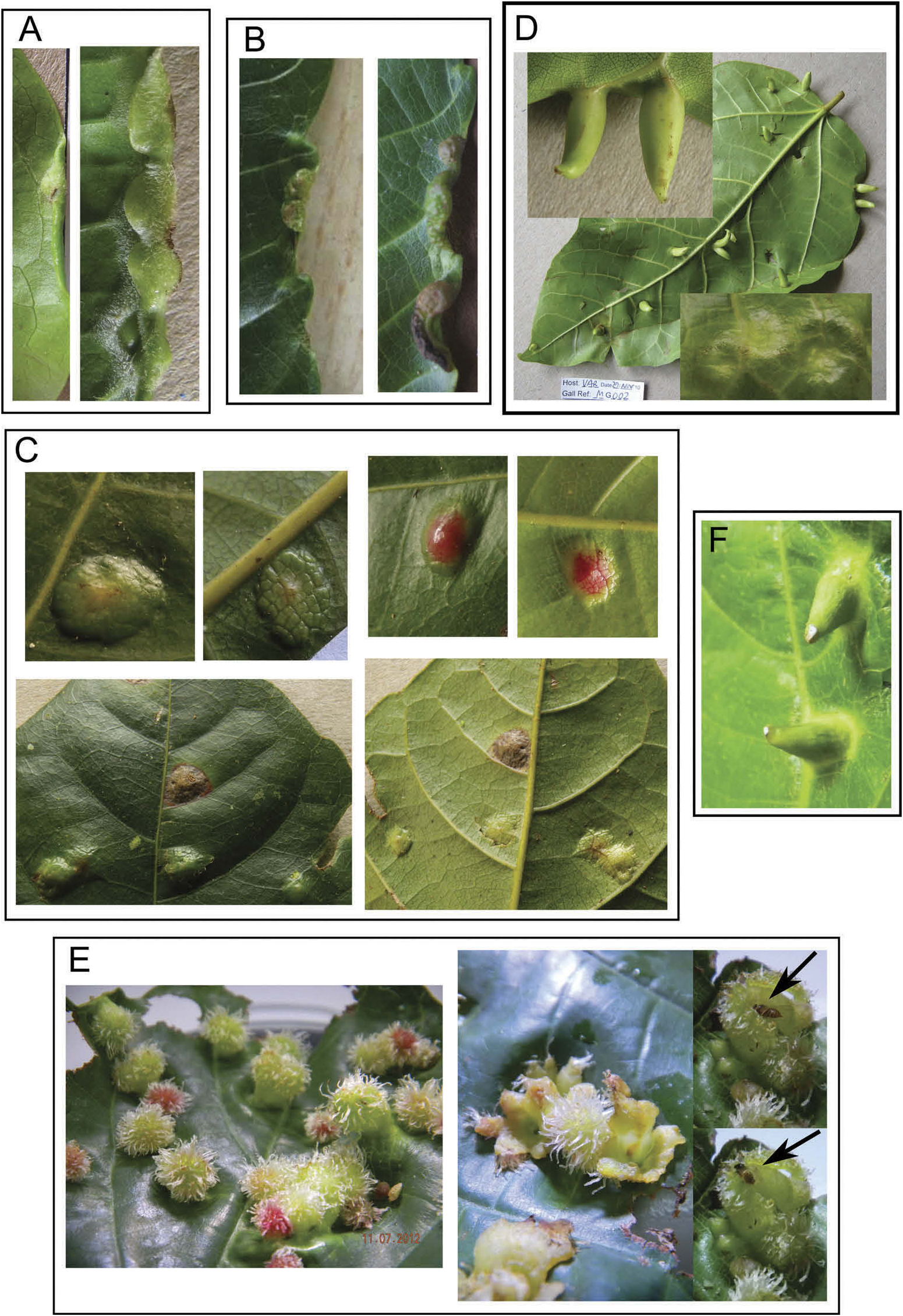
( Figures 1A – L View Figure 1 ; 2A – F View Figure 2 ; 3A View Figure 3 )
Adult colour (ethanol material)
Forewing without pattern, but membrane generally fuscous, veins light brown. Body generally mid to light brown, antennal segments 3 – 10 darker brown.
Adult structure
Forewings ( Figure 1A View Figure 1 ) long and narrow with acutely pointed apex, cell cu 1 distinctly larger than cell m 2, vein Rs short, curving to the wing margin; a group of marginal radular spines present in cells cu 1, m 1 and m 2; surface spinules sparsely scattered in all cells; wing margins and veins sparsely covered with short to minute setae. Head not strongly deflexed with genal processes short, divergent and conical, more or less symmetrical and broadly rounded at apex ( Figure 1C View Figure 1 ). Vertex more or less flat dorsally, with lateral ocelli lying on small tubercles, medial epicranial suture distinct. Antennae ( Figure 1B View Figure 1 ) long and slender, 3rd antennal segment 0.6 × head width, 10-segmented with rhinaria apically on segments 4, 6, 8 and 9, terminal segment with one apical seta medium long (~ 0.05 mm), paired with a short blunt tube-like seta (less than half the length, ~ 0.02 mm). Clypeus ( Figure 1D View Figure 1 ) triangular in lateral view, bearing two medium long setae apically. Distal segment of proboscis medium long. Dorsum of thorax with scattered short setae. Hind leg ( Figure 1G View Figure 1 ) with meracanthus well developed and straight; metatibia with a single large genual spine basally and 1+3 (typically) or 1+2 shortly stalked sclerotized apical spurs; metabasitarsus constricted medially and longer than apical tarsus. Male terminalia ( Figure 1H View Figure 1 ) with proctiger lobed basally; paramere ( Figure 1I View Figure 1 ) expanded basally into a distinct internal bulge and then tapering evenly to a darkly sclerotized apex; apical aedeagus segment ( Figure 1J View Figure 1 ) short, hooked and with an extended, sharply pointed beak. Female terminalia ( Figure 1K View Figure 1 ) short, proctiger curving downwards and slightly longer than the bluntly terminating subgenital plate, circumanal ring relatively large, 0.36 × length of proctiger, and composed of a double row of cells; ovipositor valvulae dorsales ( Figure 1L View Figure 1 ) in profile with medial bulge dorsally.
Adult measurements (mm) and ratios (1 ♂, 1 ♀)
WL: 3.12 – 3.55; HW: 0.68 – 0.73; AL: 1.85 – 2.06; GP: 0.12; PB: 0.14 – 0.15; HVW: 1.85 – 1.96; ALHW: 2.71-2.83; VLGP: 2.00 – 2.38; VLW: 0.62 – 0.83; WLW: 2.85 – 2.86; CUR: 1.37 – 1.60; MR: 0.65 – 0.73; TLFL: 1.37 – 1.47. ♂: MP: 0.22; PL: 0.22; AEL: 0.18; MSLH: 1.00; AHS: 1.83; PLSH: 1.00. ♀: FP: 0.49; FSP: 0.32; RL: 0.18; OV: 0,12; FPRL: 2.77; FPHW: 0.67; FPFSP: 1.53.
Immature structure
Body outline elongate ovoid and more or less uninterrupted ( Figure 2A, E, F View Figure 2 ). Forewing buds with pronounced humeral lobe. Antennae of 5th instar ( Figure 2C View Figure 2 ) with seven or eight segments bearing four rhinaria, one each apically on segments 3 and 5 and two on the terminal segment; 4th instar antennae 3- or 4-segmented bearing one rhinarium on 3rd segment, and two rhinaria on terminal 4th segment; 3rd instar antennae 3-segmented bearing one rhinarium on 2nd segment and one on terminal 3rd segment; 2nd instar antennae 1-segmented bearing one rhinarium. Tarsi with well-developed claws and crescent arolia ( Figure 2C View Figure 2 ). Distinct ‘ thoracic lobes ’ visible in 5th instar (see inset Figure 2A View Figure 2 ). Anus situated ventrally; circumanal ring broad and shallowly V-shaped, with a single row of elongate cells.
Immature chaetotaxy
The 2nd – 5th instars with stalked, fan-shaped setae around the margin ( Figure 2B, F View Figure 2 ), 3rd – 5th instars with dorsal surface covered in distinct pattern created by the arrangement of simple setae on round tubercle-like annuli ( Figure 2A, B View Figure 2 ; absent in the 2nd instars with the pattern becoming progressively more intricate in older instars).
Immature measurements (mm), 5th instar (n = 2)
BL: 2.09 – 2.15; BW: 1.39 – 1.42; WL: 1.24 – 1.27; CPL: 0.76 – 0.79; CPW: 1.24 – 1.27; RW: 0.26 – 0.27; HW: 0.62 – 0.67; AL: 0.28.
Host plant
Celtis philippensis (Cannabaceae)
Distribution
PNG, Madang Province.
Biology
The gall is a leaf fold at the margin of the leaf and consists of the upper adaxial surface folding down to make the gall on the lower abaxial leaf surface ( Figure 3A View Figure 3 ). The fold becomes sealed and appears to contain a single immature. There is sometimes a single fold, but more often several discrete chambers, in this case, it is not clear whether each chamber is completely sealed from the others. When mature, the gall seal opens along the intersection between adaxial and abaxial leaf surfaces. The density of galls and immatures is high with ~ 70 immatures collected from three sample sites. Rearing from galls produced five adult psyllids from two of these sites. Other insect associates found in the galls include cecidomyid larvae, and chalcid pupae and larvae ( Encyrtidae and Eulophidae ).
Etymology
The specific epithet refers to the distinct pattern on the dorsum of older immatures appearing as an encrustation formed by the tubercle-like annuli at the base of the setae. Perfect passive participle derived from the Latin verb incrustare, to have an ornamental cover.
Comments
The host plant is a common lowland rainforest tree in New Guinea and much of South East Asia (George Weiblen, pers. comm.). Six additional triozid taxa are known from the plant genus Celtis . Yang (1984) described two of these species from Taiwan, Trioza celtisae Yang, 1984 and Trioza lineata Yang, 1984 on Celtis tetrandra and Celtis sinensis , respectively. Li (2011) described an additional species from Celtis sinensis , Trioza longigenitus ( Li 2011) , and the immatures and biology for Trioza bifasciaticeltis Li and Yang, 1991 , which has free-living immatures on the lower abaxial surface of leaves. Although no biology is mentioned for the immatures of T. celtisae , this species is related to T. lineata , which is also described as having free-living immatures on the lower abaxial surface of leaves ( Yang 1984). Li (2011) placed the above species in a new genus, Metatriozidus , but this was considered artificial and synonymized with Trioza by Yang et al. (2013). A fifth Asian species, Trioza brevifrons Kuwayama, 1910 , is recorded from Korea, Japan and Taiwan. The host-plant in Korea and Japan is Celtis sinensis var. japonica ( Kwon 1983) , but the host association of the type material from Taiwan is unknown, and these specimens appear to differ from those in Korea and Japan ( Kwon 1983; Yang et al. 2013). Lastly, a South American species, Leuronota fuscata ( Laing, 1923) develops on Celtis iguanaea ( Burckhardt and Queiroz 2012, and D. Burckhardt pers. comm.). None of these Celtis -feeding triozid species appears related to Trioza incrustata sp. nov., and no clear affiliations are apparent within the Triozidae ; we therefore place this taxon within the artificially large (polyphyletic) genus Trioza Foerster, 1848 . Trioza incrustata is the first species of Trioza known to produce leaf margin galls on Celtis .
Type material
Holotype, ♂ (slide mounted), Mis village , Madang Province, PNG (5°11 ʹ S, 145°47 ʹ E, 50 m), 21 March 2011, ex Celtis philippensis , (HE06) P. Butterill leg. ( BMNH) GoogleMaps . Paratypes, 1 ♀ ( HE 07), immatures: 2 5th, 3 4th, 1 3rd, 2 2nd ( GALL 015) as for holotype ( BMNH). Other material: immatures and galls, Baitabag village (5°8 ʹ S, 145°46 ʹ E, 100 m), and Ohu village (5°13 ʹ S, 145°40 ʹ E, 200 m), near Madang, Madang Province, PNG, March 2011, ex Celtis philippensis, P . Butterill leg. ( NARI). GoogleMaps
Gene sequences
GenBank: KT588301 View Materials ( COI), KT588307 View Materials (cytB) ( PNGHE 06 – 11).
| NARI |
National Agricultural Research Institute |
| COI |
University of Coimbra Botany Department |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |