Dendrozetes jordani, Lindo, Zoe, Clayton, Marilyn & Behan-Pelletier, Valerie, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.194106 |
|
DOI |
https://doi.org/10.5281/zenodo.5657532 |
|
persistent identifier |
https://treatment.plazi.org/id/FB2887F9-C06C-EC17-FF59-4578FCF5FED7 |
|
treatment provided by |
Plazi |
|
scientific name |
Dendrozetes jordani |
| status |
sp. nov. |
Dendrozetes jordani View in CoL n. sp.
Material examined. Holotype: Adult female. Canada, British Columbia, Vancouver Island, Mt. Cain (50°13’N, 126°18’W), elevation 740–850 m, from branch tips on western hemlock ( Tsuga heterophylla (Rafn.) Sarg. ) 28 April 1996, Kevin Jordan; deposited in the Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada ( CNC), type No. 23909. Paratypes: 20 with same data as holotype; Canada, British Columbia, Vancouver Island, Mt. Cain (50°13’N, 126°18’W), from branch tips on Pacific silver fir ( Abies amabilis (Dougl.) Forb. ) 5 June 1996, Kevin Jordan and Laura Fagan; Campbell River, Montane Alternative Silvicultural Systems site (49°55’N, 125°25’W), from branch tips on Pacific silver fir ( Abies amabilis ) 28 April 1996, Kevin Jordan, Leland Humble and Neville Winchester; Upper Carmanah Valley (48°44’N, 124°37’W), from moss in canopy of Sitka spruce ( Picea sitchensis (Bong.) Carr. ), 30 August 1991, Kevin Jordan and Neville Winchester; Upper Walbran Valley (48°39’N, 124°35’W), from litter traps 1m above forest floor below western hemlock ( Tsuga heterophylla ) and western redcedar ( Thuja plicata Donn ex D. Don ), 10 January 2005, Zoë Lindo; Pacific Rim National Park (49°02’N, 125°00’W), from lichens on western hemlock ( Tsuga heterophylla ) branches, 11 October 2005, Valerie Behan-Pelletier; Clayoquot Sound (Watta 49°27’N, 126°02’W, Moyeha 49°24’N, 125°54’W, Sydney 49°30’N, 126°17’W), from moss in canopy of Sitka spruce ( Picea sitchensis ), 12 August 2007, Kevin Jordan and Zoë Lindo. USA, Washington, Quinault Rain Forest (47°32’N, 123°40’W), from moss on Bigleaf maple ( Acer macrophyllum Pursh ) branches at 15 m, 2 June 2007, Nalini Nadkarni. Paratype series divided between the CNC and the Pacific Forestry Centre of the Canadian Forestry Service, Victoria, British Columbia, Canada.
Etymology. This species is named for Kevin Jordan, who collected the type material and has contributed extensively to canopy sampling and arthropod biodiversity research in forests of Canada, Gabon, Central America, Malaysia and Samoa. Mr. Jordan has climbed an estimated 15,000 trees in his 20 year career as an “arbornaut”, primarily using single rope climbing methods.
Diagnosis. Adult. Total length 700–825 µm, with character states of Peloppiidae ( Grandjean 1954a; as Ceratoppiidae ), and character states of Dendrozetes as described above. This species can be differentiated from Dendrozetes caudatus by the presence of a single pair of prominent, knife-like posterior notogastral setae (h 1), rather than two pairs (h 1 and p 1); longitudinal ridge absent between epimeral border III and IV; prodorsal interlamellar setae longer than lamellar setae.
Description.
Adult. ( Figs. 1–9 View FIGURES 1 – 2 View FIGURES 9 A – D )
Measurements: Mean total length: females (n = 10) 810 µm (range 792–831); males (n = 5) 731 µm (range 693–752). Mean notogastral width: females (n = 10) 507 µm (range 495–515); males (n = 5) 438 µm (range 416–446).
Integument: Microtuberculate. Integument of notogaster and ventral plate with shallow foveae (Figs. 3, 4), that of prodorsum without foveae. Cerotegument microtuberculate, primarily present at dorsosejugal scissure and laterally on prodorsum (Fig. 8).
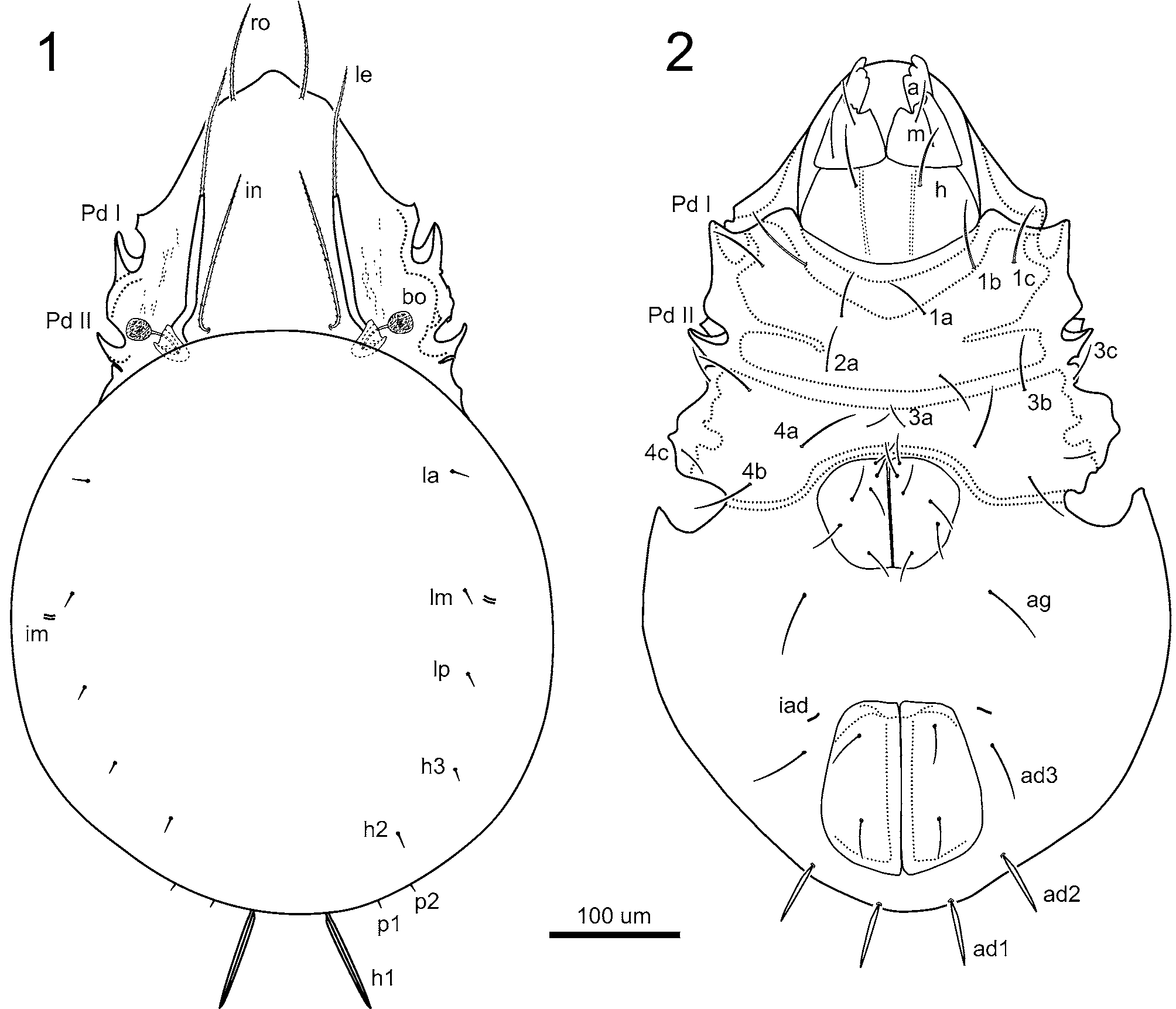
Prodorsum: Rostrum distally rounded, proximoventrally with small carina merging with rostral margin (Fig. 5). Seta ro 96–105 µm long, acuminate, barbed, straight, positioned on small tubercle, extending well anterior of rostrum. Lamellae about 128 µm long to tip of cusps. Lamellar cusps about 4–6 µm long. Seta le about 117 µm long, barbed, inserted medially on lamellar cusp and extending anteriorly beyond rostrum (Fig.
8). Seta in 185 µm long, barbed, extending anteriorly beyond tips of lamellae ( Fig. 1 View FIGURES 1 – 2 ). Mutual distance of setal pairs ro -ro, le -le, and in -in, about 60, 110, and 120 µm, respectively. Seta ex minute, evident on fewer than half of specimens examined, alveolus when present, well removed laterally from bothridial seta. Bothridial seta 48 µm long, curved medially, globose with minute barbs and longitudinal ridges, the latter only visible at high magnifications (Fig. 7); head about equal in length to stalk. Lateral aspect of prodorsum: Pedotectum I well developed, about 35 µm base to tip, broadly rounded (Fig. 8). Pedotectum II well developed, about 25 µm, triangular. Notogaster: Slightly wider than long, (ratio l:w = 0.9:1); hysterosoma of females often fattened with 2– 11 eggs of considerable size (about 237 µm long; 125 µm wide). Eight pairs of notogastral setae present, positioned peripherally ( Fig. 1 View FIGURES 1 – 2 ); la, lm, lp, h 3, h 2 and p 1, p 2 minute, simple, acuminate, 8–12.5 µm long; h 1 knife-like, prominent on the posterior contour, diverging ( Figs. 1 View FIGURES 1 – 2 , 3, 6), about 78 µm long (range 72–87 µm, n = 16), 6 µm wide, narrower basally than medially. Five pairs of lyrifissures present, im about 12 µm long ( Fig. 1 View FIGURES 1 – 2 ). Ventral region: Coxisternal setae smooth, or with few, minute barbs, relatively long; formula (epimeres I to IV) 3-1-3-3 ( Fig. 2 View FIGURES 1 – 2 ). Setae lengths as follows: 1a, 1b, 1c about 38, 54, 38 µm, respectively, 2a, 3a, 3b, 3c about 35, 30, 45, 45 µm, respectively, and 4a, 4b, 4c about 36, 42 and 35 µm, respectively. Transverse sejugal apodeme strongly developed. Minitectum along posterior border of epimere IV, extending almost to anterolateral edge of genital plate (Fig. 4). Six pairs of genital setae ranging in length from 18–32 µm, g 4 and g 5 longest. Aggenital seta about 47.5 µm long ( Figs. 2 View FIGURES 1 – 2 , 4). Two pairs of anal setae about 33 µm long. Three pairs of adanal setae; ad 3, 42 µm, simple, acuminate; ad 1 and ad 2 knife-like, about 45 µm, subequal in shape to notogastral seta h 1 ( Figs. 2 View FIGURES 1 – 2 , 4). Lyrifissure iad 15 µm long, transverse to anterior margin of anal plate, anterior to ad 3. Gnathosoma: Subcapitular mentum without tectum, with pair of sharply defined oblique carinae (Fig. 5). Seta h about 42 µm long, m and a both about 40 µm long ( Figs. 2 View FIGURES 1 – 2 , 5). Rutella atelobasic (Fig. 5). Chelicerae chelate-dentate. Legs: Ratio of leg IV to body length about 0.7:1. Approximate lengths of leg segments (femur, genu, tibia, tarsus; in µm): I 64, 53, 133, 138; II 61, 33, 55, 53; III 125, 45, 130, 70; IV 138, 63, 158, 175 ( Fig. 9 A–D View FIGURES 9 A – D ). Pretarsus heterotridactylous with large smooth empodial and lateral claws only slightly thinner. Setation (I–IV, number of solenidia in parentheses): trochanters 1-1-2-1; femora 5-4-3-2; genua 4(1)-3(1)-2(1)-3; tibiae 4(2)-4(1)-3(1)-3(1); tarsi 20(2)-15(2)-15-12; setal homologies indicated in Table 1 View TABLE 1 . Seta s of tarsus I eupathidial, anterior to (a), small, horn-like. Tarsal setae p simple, straight, short on leg I, longer and curved, almost flagellate on subsequent legs. Leg I tarsal solendia ω1 and ω2 well separated, with small famulus e close to but anterior of ω2. Leg II tarsal solendia ω1 and ω2 closely spaced but relatively short. Seta d absent from genua and tibiae of adult, except where retained bilaterally on tibia I in 2 of 14 specimens examined as minute stub in socket of solenidion φ1, as illustrated for Ceratoppia bipilis by Grandjean (1935). Porose areas on femora, tibiae and tarsi of all legs ( Fig. 9A–D View FIGURES 9 A – D ) and trochanters III, IV; those of femora and trochanters large dorsolateral, antiaxial, those of tibiae anteroventral, surrounding setae (v), those of tarsi anteroventral and proximal of seta pv ’. Solenidion σ slightly longer than tibial solenidia φ1 on leg I, and of medium length and acuminate on legs II and III; tibial solenidion φ2 subequal in length to genual solenidia.
Immatures. (Figs. 10–16)
Measurements: Mean total length: tritonymphs (n = 6) 739 µm (range 650–840); deutonymphs (n = 5) 578 µm (range 623–534); protonymphs (n = 5) 465 µm (range 445–495); larva (n = 4) 413 µm (range 366– 495); egg (n = 6) 223 µm (mode = 237, range 144–260).
Larva. Integument plicate with granular cerotegument (Fig. 14). Prodorsum: Setae ro and le, 30 and 23 µm long, respectively, slightly barbed, tapering, anteriorly placed on prodorsum, extending beyond prodorsum. Setae in about 4 µm long. Bothridial setae (bo) 27.5 µm long, with globose head, with minute barbs and longitudinal ridges. Gastronotic region: multideficient; larva with 7 pairs of setae, f 1, c 3, da, dm, dp and h 3 absent. Terminal setae h 1 about 12 µm (Fig. 14).
Protonymph and Deutonymph. As for tritonymph, except setae proportionally smaller.
Tritonymph. Integument plicate with granular cerotegument (Fig. 15). Prodorsum: rostrum rounded. Setae ro and le subequal in length (47 and 45 µm, respectively), slightly barbed, tapering, anteriorly placed on prodorsum, extending well beyond prodorsum. Setae in about 8 µm long (Fig. 12). Bothridial setae (bo) 37.5 µm long, with globose head, with minute barbs and longitudinal ridges (Fig. 12). Exobothridial setae (ex) present on all immatures examined, 7.5 µm long, subequal in shape to setae in (Fig. 12). Gastronotic region: Setae of c series, about 17 µm long, club-shaped; setae la, lm and p series of similar shape and size to c series. Terminal setae h 1 only slightly longer than other setae (about 19 µm) (Fig. 13).
FIGURES 3–8. Dendrozetes jordani n. sp., scanning electron micrograph images of adult. 3, habitus, dorsal aspect, rostral setae (ro), posterior notogastral setae (h 1); 4, habitus, ventral aspect, minitectum (mt), aggenital setae (ag), adanal setae (ad 1, ad 2, ad 3), posterior notogastral setae (h 1); 5, gnathosoma, ventral aspect, carina on lateral edge of rostrum (car), rutella (RU), setae a, m, and h; 6, habitus, lateral aspect, posterior notogastral setae (h 1); 7, detail of bothridial seta (bo) and base of interlamellar seta (in), dorsal aspect; 8, prodorsum, lateral aspect, bothridial setae (bo), lamellar setae (le), pedotectum I (PdI), pedotectum II (PdII). Scale bars = 100 µm (Figs. 3–6, 8) and 10 µm (Fig. 7).
Setae (Roman) and solenidia (Greek) are listed opposite the instar in which they first appear; () parentheses indicate pairs of setae; [] brackets indicate setal loss.
*Seta d coupled with solenidion present on fewer than 15% of specimens observed (see text).
Legs: Monodactylous. Seta d coupled with solenidion and present to tritonymph on genua I to III and tibiae I to IV in all immatures ( Table 1 View TABLE 1 ). Shape of genual solenidion σ short, baculiform and subequal in length to seta d in immatures. Tibial solenidion φ2 almost as long as seta d in deutonymph and tritonymph. Porose areas developed on femora I to IV and trochanters III and IV in the tritonymph, not evident in larva to deutonymph.
Genetic analysis. Six specimens of Dendrozetes jordani were successfully sequenced for COI (>200 base pairs) (Table 2). Specimens of Dendrozetes jordani (n = 6) had an average genetic distance among conspecifics of 2.6%. The single specimen collected from Olympic National Park, Washington State had the highest genetic distance from other conspecifics having>5% genetic separation from the five specimens collected from Pacific Rim National Park, Vancouver Island. All sequences are available from BOLD Systems (http://www.barcodinglife.org).
Ecology. Adults and immatures of Dendrozetes jordani were found on 100% of the branch clipping samples (n = 114), but only on 54% of lichen samples (n = 78) collected from the MASS site on Vancouver Island, BC. The mean abundance of D. jordani was significantly greater in western hemlock trees compared to Pacific silver fir, and significantly greater on branch samples than in lichen samples (Table 3), due to high abundance of immature D. jordani on hemlock branch tips compared to other locations. Overall immature abundance was greatest in the May sampling compared to July or November sampling ( Fig. 17 View FIGURE 17 ). Adult abundance tended to be fairly stable throughout the growing season among habitat types and tree species, yet there was a significant time-by-habitat interaction whereby average adult abundance increased in the lichen samples while decreasing in branch tips over the growing season ( Fig. 17 View FIGURE 17 ).
TABLE 2. Collection locations and habitat notes on Dendrozetes jordani specimens successfully sequenced for cytochrome oxidase I (COI). Specimen collection locations and habitat notes are given. TABLE 3. Results of repeated measures MANOVA on the abundance of adult and immature Dendrozetes
jordani n. sp. collected from branch tip and lichen samples (habitat type) in Pacific silver fir and western
hemlock trees over three sampling periods.
Systematics. When first described, Dendrozetes , with D. caudatus Aoki as its type species, was tentatively placed in the Eremaeidae because of the parallel lamellae ( Aoki 1970). However, Aoki (1970) noted the similarity with members of the Peloppiidae , a placement maintained by Balogh & Balogh (1992), and Subías (2004, 2009). This placement is further confirmed by the discovery of adults and immatures of D. jordani described above, which allow a more complete diagnosis of the genus and confirmation of familial character states of Peloppiidae ( Grandjean 1954a) . However, it should be noted that diagnostic character states for immature Dendrozetes are based only on D. jordani . There are discrepancies between the diagnosis above and that of Aoki (1970). We examined the type series of D. caudatus and the epimeral setation is 3-1-3-3 rather than 3-1-2-2. Porose areas are present on tibiae and tarsi I to IV of the type series, in the same position as in D. jordani .
FIGURES 10–16. Dendrozetes jordani n. sp., scanning electron micrograph images of immature stages. 10, tritonymph, lateral aspect; 11, larvae and eggs within body cavity of dissected female, dorsal aspect; 12, nymph, detail showing bothridial seta (bo), interlamellar seta (in), and exobothridial seta (ex); 13, nymph-nymph molt, lateral aspect, posterior notogastral setae (h 1); 14, larva, lateral aspect, posterior notogastral setae (h 1); 15, nymph-nymph molt, dorsal aspect; 16, nymph, dorsal aspect. Scale bars = 100 µm (Figs. 10, 11, 13–16) and 10 µm (Fig. 12).
Nymphs of Dendrozetes jordani n. sp. are apheredermous and lack dorsocentral setae, as do those of described nymphs of other Peloppiidae— Metrioppia helvetica Grandjean (Grandjean 1931) , Ceratoppia ( Grandjean 1954a) —and of Liacaridae ( Arlian & Woolley 1970; Travnícek 1977, 1982a, 1982b). In contrast, other gustavioid families with described immatures, Tenuialidae and Gustaviidae , have eupheredermous nymphs ( Grandjean 1954a, Norton 1983). Whereas nymphs of Ceratoppia are quinquedeficient, with loss of setae f1, da, dm, dp, and p3, those of D. jordani are multideficient with the additional loss of c3.
Larvae of all gustavioid taxa so far described— Liacarus cidarus Woolley (Arlian & Woolley 1969) , L. coracinus (C. L. Koch) , L. subterraneus (C. L. Koch) (Travicnek 1977, 1982a)—have the dorsocentral setae da, dm, dp; these do not develop in D. jordani . To our knowledge, this absence is unique in described larvae of Brachypylina . We have no plausible explanation for the absence of these setae. There is no evidence that larvae are short-lived (only 5 of 25 larvae examined were in pre-moult). The larva is a feeding stage, with 18 of 25 larvae containing food.
Gravid females carried an average of seven eggs. Two out of 14 gravid females examined showed both eggs and prelarva within the body cavity (2 prelarva / 7 eggs); the prelarval stage was evident by the presence of the laterofrontal groove and/or Claparède’s organ ( Grandjean 1954b). In one case larvae were observed within the body cavity of a dissected gravid female (see Fig. 11), however we do not know whether the female was collected alive (suggesting possible larviparity) or dead, suggesting the larva likely developed within the body cavity following death. Previous reports of larviparity (larva hatching within adult body cavity and deposited in active larva stage) are uncommon in Oribatida ( Norton 1994) , and ovovivipartity (larva hatch immediately following deposition) and mixed-parity reproduction modes are also infrequently reported ( Haq et al. 1991; Søvik 2003).
Ecology. The habitat of Dendrozetes jordani is similar to that of the type species for the genus, Dendrozetes caudatus , i.e., arboreal habitats and litterfall associated with tree species primarily from the family Pinaceae ( Aoki 1970; Aoki 1973; Ito 1986; Fujikawa et al. 1993). Additionally, while many arboreal oribatid mite species are collected in association with epiphytic cover such as moss, lichen and suspended soils ( Seyd & Seaward 1984; Behan-Pelletier et al. 1993; Lindo & Winchester 2007), D. jordani was collected in high densities on branch tips, with lower observed abundance within lichens on branches (see also Winchester et al. 2008). This evidence suggests that Dendrozetes may be host-tree dependant.
Peloppiidae is well represented in North America ( Marshall et al. 1987), however, Dendrozetes had not been previously recorded from outside of Japan. As Dendrozetes jordani represents the first record of Dendrozetes in North America, further sampling of trees in Pinaceae , particularly Tsuga species, may uncover additional species or distribution records.
The abundances of Dendrozetes jordani adults and immature instars throughout the active growing season indicate that the populations studied have continuous reproduction throughout the season, or possibly two hatching events per year. Further studies are needed to confirm seasonal trends and distributional patterns of adults and immatures among branch and lichen habitats.
TABLE 1. Development of leg setiform organs in Dendrozetes jordani n. sp.
| Leg I Larva Protonymph Deutonymph Tritonymph | Trochanter - - - v’ | Femur d bv ’’ - (l) v’ - | Genu d (l) σ - v’ - | Tibia d (l) v’ φ1 - φ2 v’’ | Tarsus (ft)(pl)(tc)(p)(u) s (a)(pv) e, ω1 ω2 - - |
|---|---|---|---|---|---|
| Adult Leg II Larva Protonymph Deutonymph Tritonymph Adult Leg III | - - - - v’ - | - d bv ’’ - (l) - - | - d l’ v’ σ - - v’’ [d] | [d]* d l’ v’ φ - l’’ v’’ [d] | (it) (v) (p)(tc)(ft)(u) s (a)(pv) ω1 - ω2 - (it) |
| Larva Protonymph | - - | d ev’ - | d l’ σ - | d v’ φ - | (p)(tc)(ft)(u) s (a)(pv) - |
| Deutonymph | v’ | l’ | - | l’ | - |
| Tritonymph Adult Leg IV | l’ - | - - | v’ [d] | v’’ [d] | - (it) |
| Larva Protonymph | - - | - - | - - | - - | - (p) ft’’ (u)(pv) |
| Deutonymph | - | d ev’ | d l’ | d v’ φ | (tc)(a) s |
| Tritonymph Adult | v’ - | - - | v’ - | l’ v’’ [d] | - - |
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |