Conothele Thorell, 1878
|
publication ID |
https://doi.org/10.11646/zootaxa.4984.1.22 |
|
publication LSID |
lsid:zoobank.org:pub:26314FBC-18A7-4EBA-B0CF-5ADD5B03F5FD |
|
DOI |
https://doi.org/10.5281/zenodo.5206069 |
|
persistent identifier |
https://treatment.plazi.org/id/FB3B8783-2522-FFB9-FF7E-F904FD95FABA |
|
treatment provided by |
Plazi |
|
scientific name |
Conothele Thorell, 1878 |
| status |
|
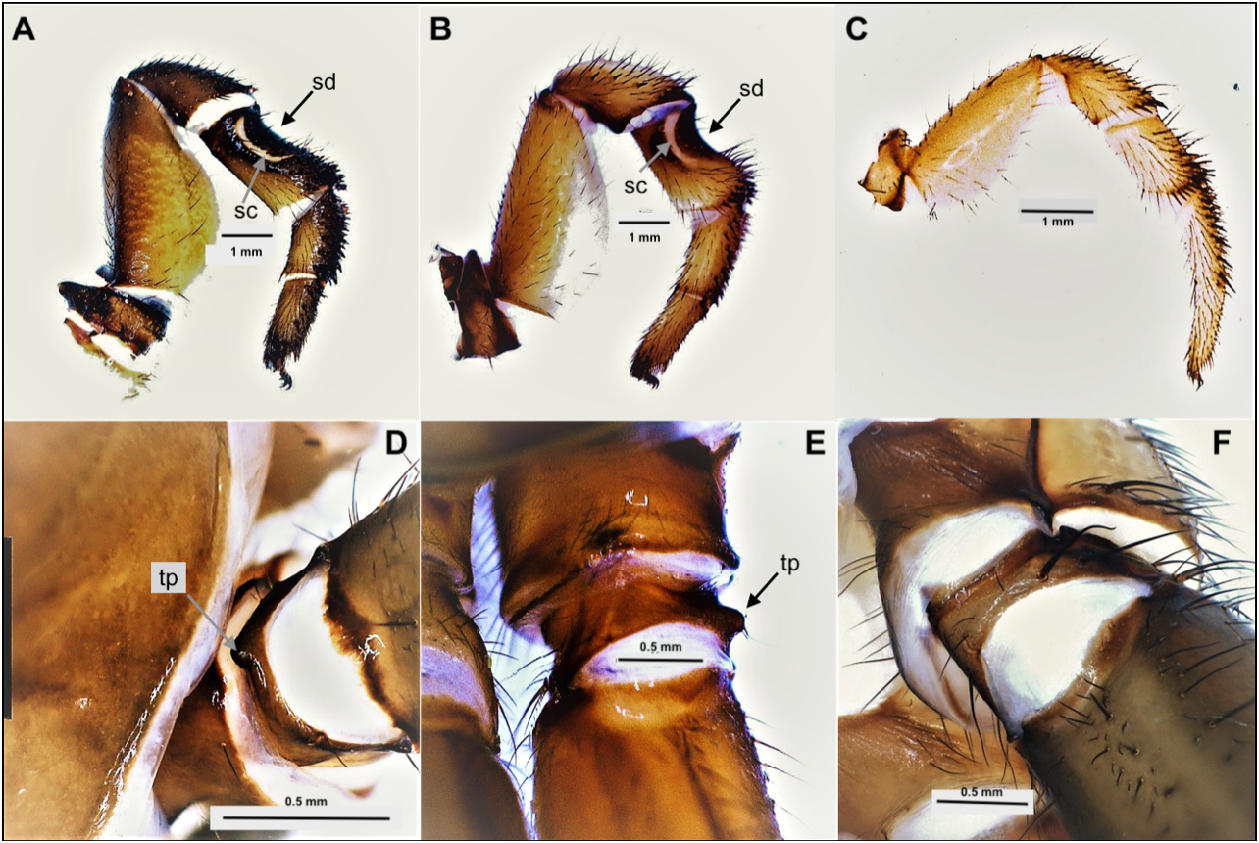
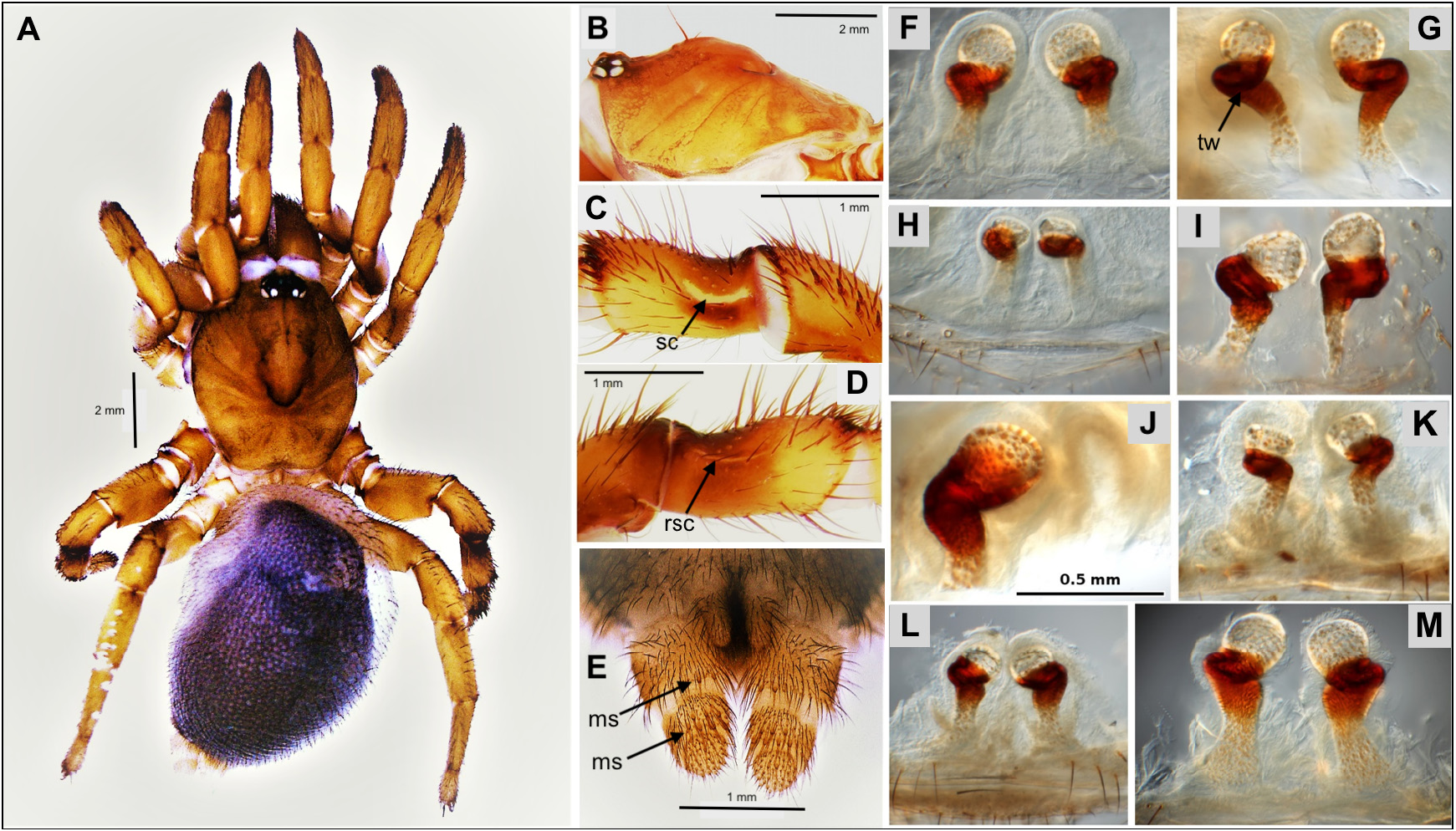
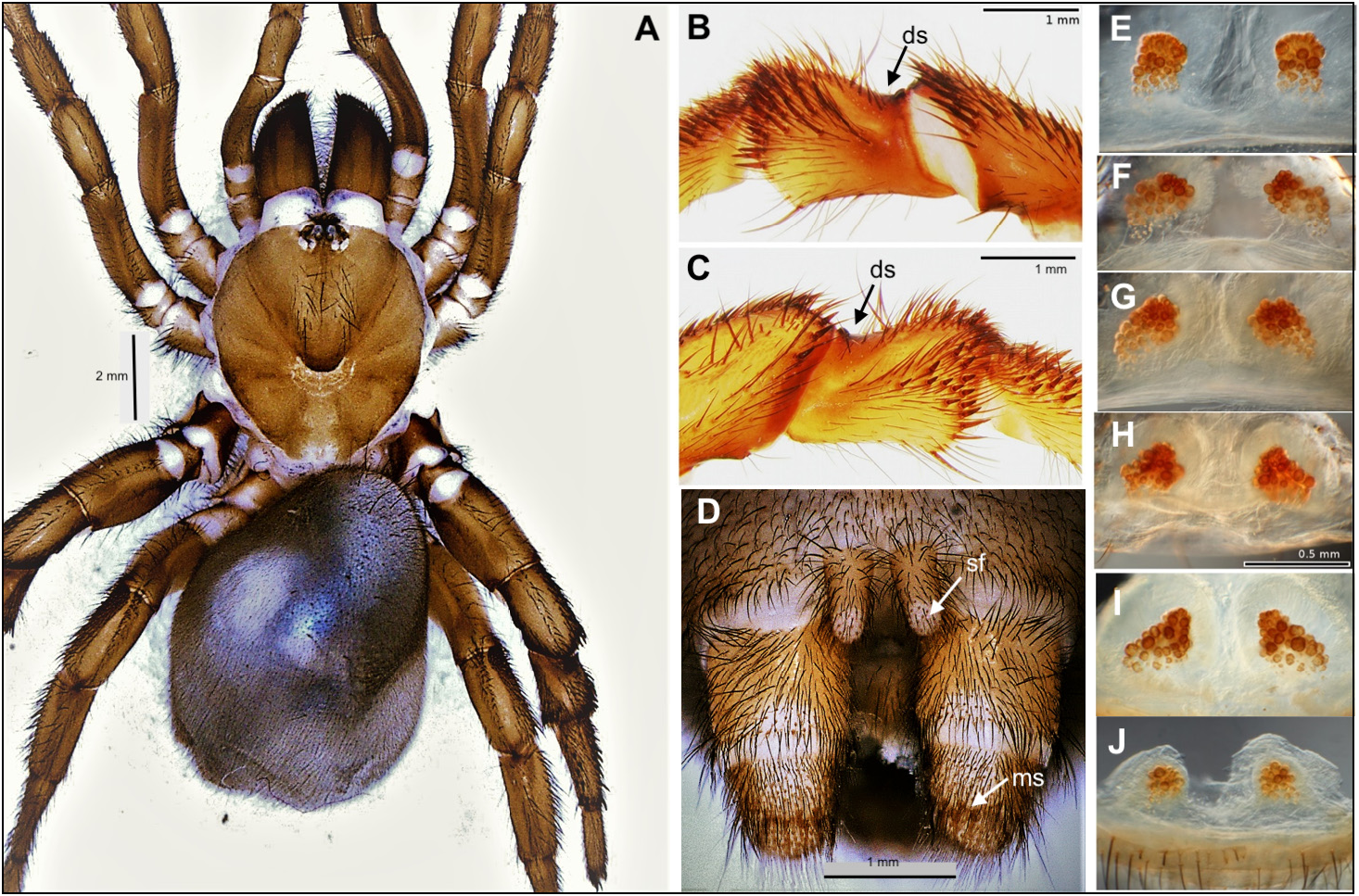
Diagnosis. As mentioned above, Conothele is readily distinguished from Latouchia by the long, thin and flexible embolus of the male palpal organ ( Figs 4 View FIGURES 4 H−K, 6H−K cf. Figs 8 View FIGURES 8 F−I, 10 F−I), by the tripartite spermathecae ( Figs 5 View FIGURES 5 F−K, 7F−H cf. Figs 9 View FIGURES 9 E−J, 11E−L), by the saddle-shaped dorsal depression with saddle-crescents in tibia III (best seen in females; Figs 3 View FIGURES 3 A−B, 5 C−D, 7C−D cf. Figs 3C View FIGURES 3 , 9 View FIGURES 9 B−C, 11 B−C), and by the presence of a prodorsal protuberance on trochanter III (absent in Latouchia , Figs 3 View FIGURES 3 D−E cf. Fig. 3F View FIGURES 3 ).
Notes. Distinguishing Conothele from Ummidia has been the subject of a long-standing debate in which three views, leading to two opposing hypotheses, are expressed in the literature. The first view, mainly based on data from morphology, behaviour and distribution, claims that the two genera are synonymous ( Main 1957, 1985, 1998; Decae 2010). The second view, based on molecular studies, claims that Conothele and Ummidia are separate, although reciprocally monophyletic genera, that are separated in space and in time ( Godwin et al. 2018; Opatova et al. 2019: fig. 5; Godwin & Bond 2021). The third view, based on differences in burrow or nest structure, claims that Conothele and Ummidia are distinct in their behaviour ( Haupt 2006).
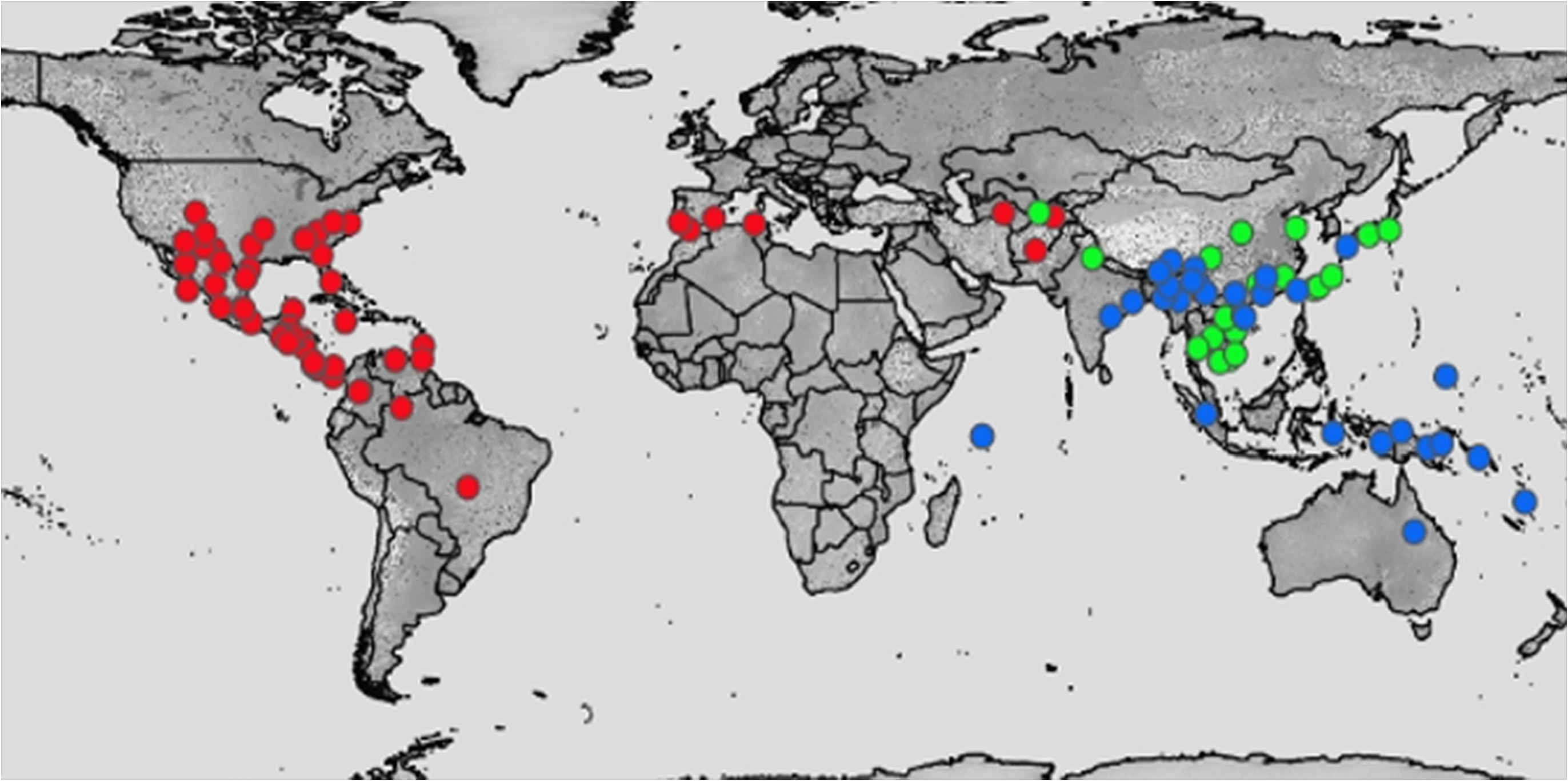
Arguments supporting the first view are: the similarity in morphology and behaviour of Conothele and Ummidia ( Main 1957, 1985, 1998; Decae 2010), the fact that there is no unequivocal distributional divide to separate the genera in space ( Fig.1 View FIGURE 1 ) and the fact that the two genera are consistently found to constitute a monophyletic group in DNA-based phylogenetic studies ( Hedin & Bond 2006; Bond et al. 2012; Opatova et al. 2013; Wheeler et al. 2016; Opatova et al. 2019). Arguments supporting the second view are based on the supposedly non-overlapping distributions of the two genera and phylogenetic analysis using hundreds of loci, resulting in the conclusion that Conothele and Ummidia are two independent evolutionary lineages that are to be regarded as reciprocally monophyletic ( Godwin et al. 2018; Godwin & Bond 2021).
An important difference between these two views is that the first view, advocating for synonymy, rests on data that were collected in over two centuries of descriptive work which revealed the intercontinental distribution of Ummidiinae shown in Fig. 1 View FIGURE 1 , while the DNA-based second view, advocating for distinction, rests on recent sampling of populations from a few widely separated localities almost at opposite ends of the geographical range of Ummidiinae ( Godwin et al. 2018: tab. 1, fig. 1).
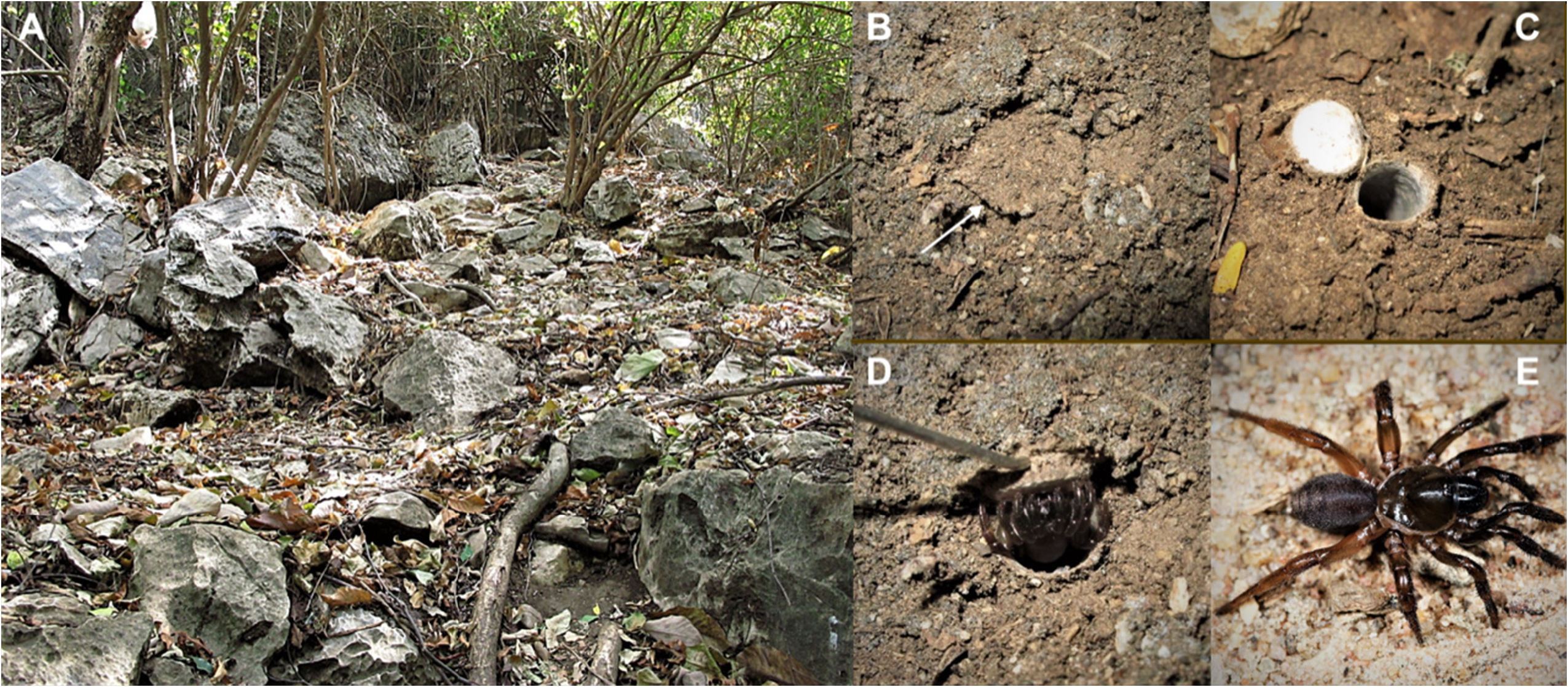
The third view is here rejected on grounds of personal observations on a wide range of nest/burrow structures in many Conothele species in Southeast Asia and of Ummidia species in Central America and the western Mediterranean. Instead of finding different nest/burrow types in the two genera, our observations revealed a remarkable overlap in nest/burrow types for Conothele and Ummidia . Most species in both genera construct simple, dead-ended underground burrows of variable length, that are densely lined with thick white silk and covered by a thin, stiff, almost circular hinged trapdoor ( Figs 14 View FIGURES 14 B−D). Other species, in both genera, construct terrestrial burrows, that are furnished with a second trapdoor at the bottom of the burrow opening to an underground cavity. These remarkable burrows are very similar to burrows found in the ctenizid genus Cyrtocarenum ( Decae 1996) . In Conothele and Ummidia these ‘two-door burrows’ are found in geographically widely separated species such as U. algarve from Portugal ( Decae 2010) and C. varvati from India ( Pickard-Cambridge 1907; Siliwal et al. 2009). A second trapdoor at the bottom of the burrow was also observed in an unidentified Conothele female collected at the type locality of C. isan spec. nov. in Thailand.
Furthermore, there are species, such as Conothele arboricola from the southwest Pacific ( Pocock 1898b), an undescribed Conothele species from Koh Tao, a small island off the east coast of southern Thailand, another undescribed Conothele species from the Palau Islands, and an as yet undescribed Ummidia species from Central America, that construct short cigar-shaped trapdoor-nests attached to trees trunks well above ground level. These nests are similar to arboreal tree nests found in the barychelid genus Sason (e.g., Schwendinger 2003: fig. 15) and the Migidae ( Griswold & Ledford 2001) .
As mentioned above, the lack of diagnostic characters in morphology, behaviour and genetics has led to the use of geographical separation as an argument to distinguish genera and species in Ummidiinae ( Xu et al. 2017; Yang & Xu 2018; Liu et al. 2019; Godwin & Bond 2021). Geography-based arguments to structure taxonomy are, however, debatable, particularly when used to classify taxa such as Conothele and Ummidia which are not as strongly dispersal-limited as most other mygalomorph spiders ( Bond et al. 2012). The large geographical ranges observed in Conothele and Ummidia have been attributed to their capacity for aerial dispersal ( Main 1985, 1998; Decae 2010; Opatova et al. 2013, 2019). Aerial dispersal has been established or inferred in several Ummidia and Conothele species ( Baerg 1928; Main 1985; Coyle 1985; Coyle et al. 1985; Eberhard 2006; Fisher et al. 2014). Aerial dispersal in mygalomorph spiders however, appears much less effective than in araneomorph spiders ( Opatova et al. 2016) and can therefore not explain long-distance or over-water dispersal events that have apparently shaped the distribution ranges of Conothele and Ummidia . Another, possibly stronger explanation for the wide geographical ranges and the presence of Ummidia and Conothele on volcanic and remote oceanic islands ( Simon 1891a; Pocock 1898a, b; Main 1957; Roewer 1963; Saaristo 2002; Decae 2010) is dispersal of principally arboreal species by rafting. For the arboreal Conothele and Ummidia species mentioned above, dispersal events as described for species of the migid genus Moggridgea ( Harrison et al. 2017) can be envisioned.
In conclusion: it is clear that sampling the entire geographical range and collecting biological information of Ummidiinae is necessary to build a satisfactory understanding of this still poorly known, but remarkably successful and diverse group of trapdoor spiders.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Ummidiinae |