Phytoptus hirtae Roivainen 1950
|
publication ID |
https://doi.org/ 10.5281/zenodo.200512 |
|
DOI |
https://doi.org/10.5281/zenodo.6183877 |
|
persistent identifier |
https://treatment.plazi.org/id/FC139970-4F2A-8247-FF73-FC8EFE3BF867 |
|
treatment provided by |
Plazi |
|
scientific name |
Phytoptus hirtae Roivainen 1950 |
| status |
|
Phytoptus hirtae Roivainen 1950
( Fig. 5 View FIGURE 5 )
Roivainen 1950:13,14 Fig. 9
non Skoracka et al. 2004:7, 8; no fig. Petanović et al. 2007:110; no fig.
Protogyne female. Body vermiform, whitish, 393 long, 67 wide. Prodorsal shield semicircular with five distinct lines (median, two admedians and two submedians-I) extending from rear to anterior shield margin. Median line with a gap in anterior one fourth. Admedian lines are parallel to median line in anterior third of shield; diverging in rear two thirds of shield, forming a figure resembling an inverted Greek letter “ψ”. Two short lines present between median and admedian lines near rear shield margin. Submedian-I lines straight in anterior half of shield and sinuate in rear half. Two short lines present between admedian and submedian-I lines in anterior third of shield. Submedian-II lines from anterior shield margin running back past inner side of tubercules of ve and ending well in front of tubercules of sc. These lines may consist of 2 to 3 fragments or may be entire with a small curve at rear. Two to three short lines present on lateral sides of shield. Surface of prodorsal shield before ve with small granulations. Prodorsal shield 37 long; ve 13 long, directed forward, tubercles 28 apart; sc 3 long, directed backward, tubercles 23 apart. Distance between tubercules ve and sc 22. Gnathosoma 26 long. Leg I 37 long, tibia 8, l' 3, tibial solenidion 9, tarsus 7, ω 11 long, without knob, empodium 7/6-rayed. Leg II 33 long, tibia 7, l' absent, tarsus 6 long, ω 11 long, without knob, empodium 7/6- rayed. Setae bv present. Short longitudinal line present near tubercule of bv on both femora. Coxae with numerous oval microtibercules. Suboral plate absent. Setae 1b 19 long, 19 apart; 1a 23 long, 15 apart; 2a 56 long, 36 apart. Epigynium smooth, 12 long, 22 wide; 3a 13 long. Opisthosoma with 87 microtuberculated annuli. 3 annuli present before epigynium. Setal lengths: c1 108, c2 44, d 26, e 9, f 51, h1 5; 9 annuli between rear shield margin and tubercules of c1, 7 annuli anterior to c2; 17 annuli situated between c2 and d; 29 annuli situated between d and e; 24 annuli situated between e and f, 10 annuli situated between f and h1.
Male. In comparison to females, males are smaller in size with shorter legs and opisthosomal setae and possess a 6/5-rayed empodium. The design of the male prodorsal shield is similar to that of the female. Measurements of males are given in Table 4 View TABLE 4 .
Material examined. 10 protogyne females and 5 males (slide #28-09) from Carex hirta L. ( Cyperaceae ) [inside leaf sheaths; no damage was observed] RUSSIA: Pskov Prov., Loknya area, vill. Gogolevo, meadow near pine-tree forest; 57°00'22'' N, 30°56'36'' E, 14 July 2009, coll. P. E. Chetverikov.
Additional material. 22 protogyne females, 7 males and 8 nymphs (slides #26-09, #27-09), same host, date, locality and coll. as before; 29 deutogyne females, 2 protogyne female, 1 overwintering male and 12 nymphs (slides #29-10, #30-10, #31-10) same host, date, locality and coll.; 6 protogyne females and 3 nymphs (slides #27- 0 4, #28-04), same host and coll., RUSSIA: Smolensk Prov., Demidov area, vil. Kobysi, 55°26'01'' N, 31°22'74'' E, 10 July 2004; 5 protogyne females and 3 nymphs (slides #44b-04), same host and coll., RUSSIA: Pskov Prov., Nevel area, vil. Turichino, 55°85'70'' N, 29°57'03'' E, 16 July 2004; 28 females and 3 nymphs (slides #22-02, #23- 0 2, #24-02), same host and coll., RUSSIA: Leningrad Prov., Gatchina area, Marienburg, 59°57'57'' N, 30°08'54'' E, 0 8 October 2002; 12 females and 2 nymphs (slide #92-03), same host, locality and coll., 21 September 2003; 24 protogyne females and 7 nymphs (slides #108-02, #112-02), same host and coll., UKRAINE: Dnepropetrovsk, on bank of Lake Moskovskoye, 48°51'38'' N, 34°97'47'' E, 12 August 2002; 7 protogyne females, 1 male and 2 nymphs (slides #56-04, #57-04, #58-04, #59-04), same host, locality and coll., 11 August 2008; 1 protogyne female (slide #1093/7), same host, SERBIA: Belgrade, near Ostružnički most, 0 8 May 2007, coll. D. Smiljaniċ.
Character Mite species
Mite species
P. hirtae P. dehesae P. liroi * see remarks for Table 5
Differential diagnosis. P. hirtae is close to Phytoptus dehesae Roivainen 1953 , P. l i ro i Roivainen, 1947 and P. atherodes sp.n. P. hirtae , P. dehesae and P. l i ro i differ in the length of the sc, body measurements, number of dorsal annuli and length of the median line ( Table 7 View TABLE 7 ). The differences between P. hirtae and P. atherodes are mentioned above in the differential diagnosis of P. atherodes .
Distribution and host plants. Specimens of P. h i r t a e were found inside leaf sheaths of Carex hirta L. 1753, in Sweden and Serbia ( Roivainen 1950; Petanoviċ et al. 2007). Since 2002, I repeatedly found P. h i r t a e on the same host in Northwest Russia and the Ukraine ( Fig. 3 View FIGURE 3 ) where this mite species is quite common. The host plant for this mite species is also found in Northern Africa, Iran and Turkey ( Egorova, 1999), so future surveys may reveal a wider distribution for P. hirtae .
Remarks. According to Skoracka et. al. (2004), P. h i r t a e also lives on Carex arenaria L. and Carex colchica subsp. ligerica (J. Gay) Egor., 1973 in Poland. On the same host-plants in Sweden about 60 years ago, Roivainen (1950) found Phytoptus liroi Roivainen 1947 . Since 2002 in the Ukraine and European part of Russia, I have regularly collected mites of the genus Phytoptus from C. arenaria and C. colchica . Morphologically, these specimens were much closer to P. l i ro i than to P. hirtae . Sedges C. arenaria , C. colchica and C. hirta belong to different subgenera of the genus Carex (the first two species belong to subgenus Vignea whereas the third belongs to the subgenus Carex ) and phylogenetically are rather remote from each other ( Egorova 1999). In Russia and Ukraine, I found P. h i r t a e only on C. hirta . Therefore, I suggest that Skoracka et al. (2004) probably collected a different species of Phytoptus (probably P. l i ro i) in Poland on C. arenaria and C. colchica .
Deuterogeny of Phytoptus hirtae . Protogyne and deutogyne females of P. h i r t a e (10 of each from samples #9 & #10; Table 2 View TABLE 2 ) collected in the same meadow near the village of Gogolevo, Russia, were measured and compared to reveal the existence of deuterogeny. Generally, summer and overwintered females are identical except for differences in the length of setae: overwintered females have longer setae ve, e, f and shorter coxal setae 1b ( Table 4 View TABLE 4 ). Moreover, in comparison to uncoloured, whitish summer females, overwintering females are yellowish.
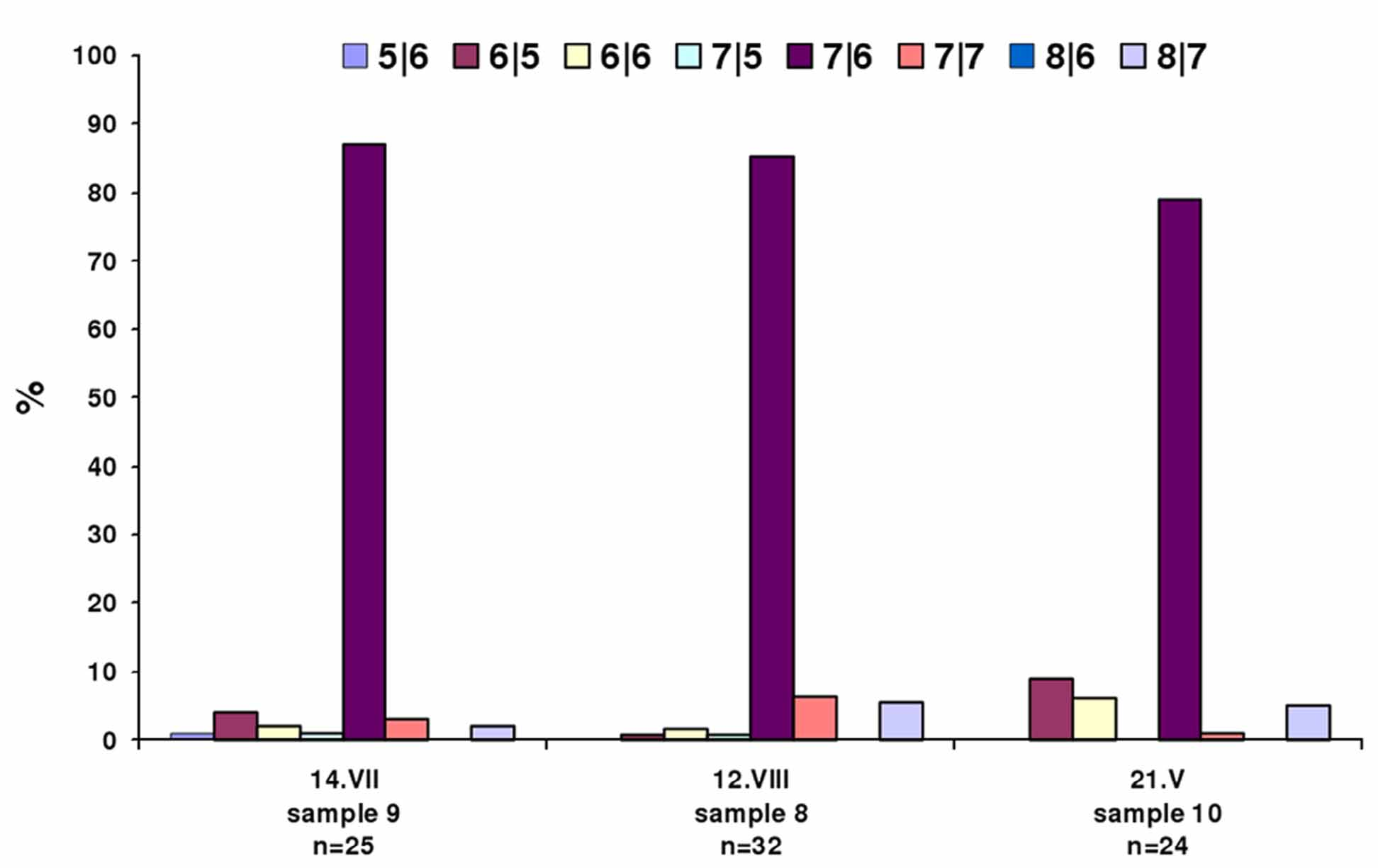
Variability of the empodial ray number of P. h i r ta e. I counted the number of rays on the inner and outer margins of the empodia on legs I and II of 81 females (from samples #8, #9 and #10; Table 2 View TABLE 2 ). The frequency of different variants of empodial numbers were calculated and plotted ( Fig. 6 View FIGURE 6 ). I have concluded that:
1) in the same sample, mites have different numbers of empodial rays (5/6, 6/5, 6/6, 7/5, 7/6, 7/7, 8/7);
2) one and the same mite may have different empodia which differ in the number of rays. For example, 6/5-6/6-6/ 5-5/5;
3) 7/6-rayed empodia are the most common form;
4) the frequency of 7/6-rayed empodia is constantly high: 85 to 87% among summer females and 79% among overwintered females;
5) the frequency of 5/6-, 7/5- and 8/6-rayed empodia is constantly low (1-2%);
6) 6/5- and 6/6-rayed empodia are rather rare among summer females (<4%) whereas among overwintered females, such variations of the empodia can be twice or thrice the number (9% and 6%, respectively). I consider 5/6-, 6/5-, 6/6-, 7/5- and 8/6-rayed empodia to be aberrant forms.
So, females of P. hirtae do not demonstrate such a distinct seasonal variability in the number of empodial rays as do females of P. atherodes . But in comparison with summer females, two tendencies are peculiar for the overwintered females of P. hirtae : a slight decrease in the frequency of the most common variant of the empodium (7/6) and an increased frequency of the aberrant forms of empodia with reduced numbers of rays (6/5 and 6/6). See Fig. 6 View FIGURE 6 .
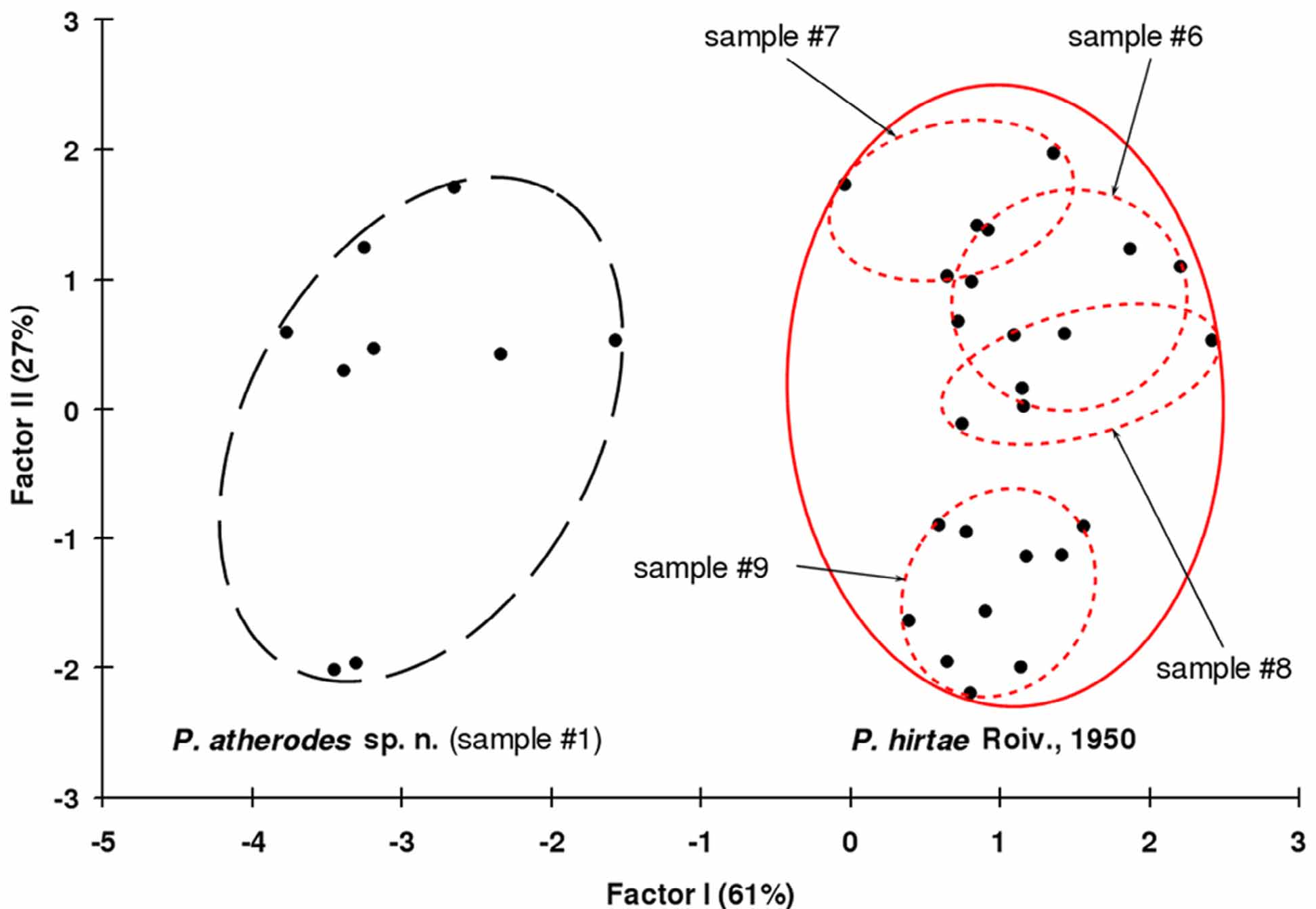
Intraspecific and interspecific morphological variability of P. atherodes and P. h i r ta e. These two mite species are very similar morphologically, moreover though, P. hirtae from geographically isolated populations differs slightly in some characters but most measurements overlap (Table 5). Principal component analysis (PCA) of the samples #1, #6, #7, #8 and #9 ( Table 2 View TABLE 2 ) was carried out to compare morphological variability of protogyne females of P. hirtae and P. atherodes by a complex of characters. Two main Factors I & II ( Jolliffe 2002, StatSoft, Inc. 2011), representing together about 90% of total morphological dispersion, were revealed ( Fig. 7 View FIGURE 7 ). Samples ##2 to 5 which included many deutogynes were not analysed to avoid the accidental confusion between deutogynes and protogynes. Characters weakly correlated (r≤0.5) with Factors I and II were excluded from analysis. As a result, a complex of 6 characters was formed based on the number of empodial rays, length of ve, c2, d, sc and distance between tubercules of ve. The first 4 characters had a high correlation with factor I (r≥0.9) and the other two were highly correlated with factor II (r≥0.8). Graphically, the samples belonging to different species were distinctly separated by factor I ( Fig. 7 View FIGURE 7 ) which reflects interspecific variability. This fact may be a supplemental argument to the validity of the separation of the species P. hirtae and P. atherodes . Samples including P. h i r t a e differed by meaning of Factor II ( Fig. 7 View FIGURE 7 ). So that Factor II, reflects the intraspecific variability (variability between different populations) of this mite species.
TABLE 7. Morphological differences between P. hirtae, P. dehesae and P. liroi
| Character | This paper | Roivainen 1950 | Roivainen 1953 | Roivainen 1947, 1950 |
|---|---|---|---|---|
| Median line of pro- dorsal shield | From rear shield margin to anterior shield margin; with a gap anteriorly | From rear shield margin to anterior shield margin with a gap anteriorly | From rear shield margin to anterior shield margin with a gap anteriorly | Present in rear 2/3 of prodorsal shield |
| Number of dorsal annuli | 81–91 | 100–105* | 70 | 77–92 |
| Length of body | 282–414 | 355–390 | 230 | 310–400 |
| Length of sc (s.d.2) | 2(1–3) prickle-shaped | 4–5 prickle-shaped | 8–9 | 5–10 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.