Characidium onca, Melo & Ribeiro & Lima, 2021
|
publication ID |
https://doi.org/ 10.1590/1982-0224-2020-0061 |
|
publication LSID |
lsid:zoobank.org:pub:7A74DAE7-2100-4D64-8A5A-B6D01F888CFD |
|
DOI |
https://doi.org/10.5281/zenodo.10998962 |
|
persistent identifier |
https://treatment.plazi.org/id/A4755AF5-77AF-46FA-B034-49DD4A46D2B4 |
|
taxon LSID |
lsid:zoobank.org:act:A4755AF5-77AF-46FA-B034-49DD4A46D2B4 |
|
treatment provided by |
Felipe |
|
scientific name |
Characidium onca |
| status |
sp. nov. |
Characidium onca , new species
urn:lsid:zoobank.org:act:A4755AF5-77AF-46FA-B034-49DD4A46D2B4
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ; Tab. 1 View TABLE 1 )
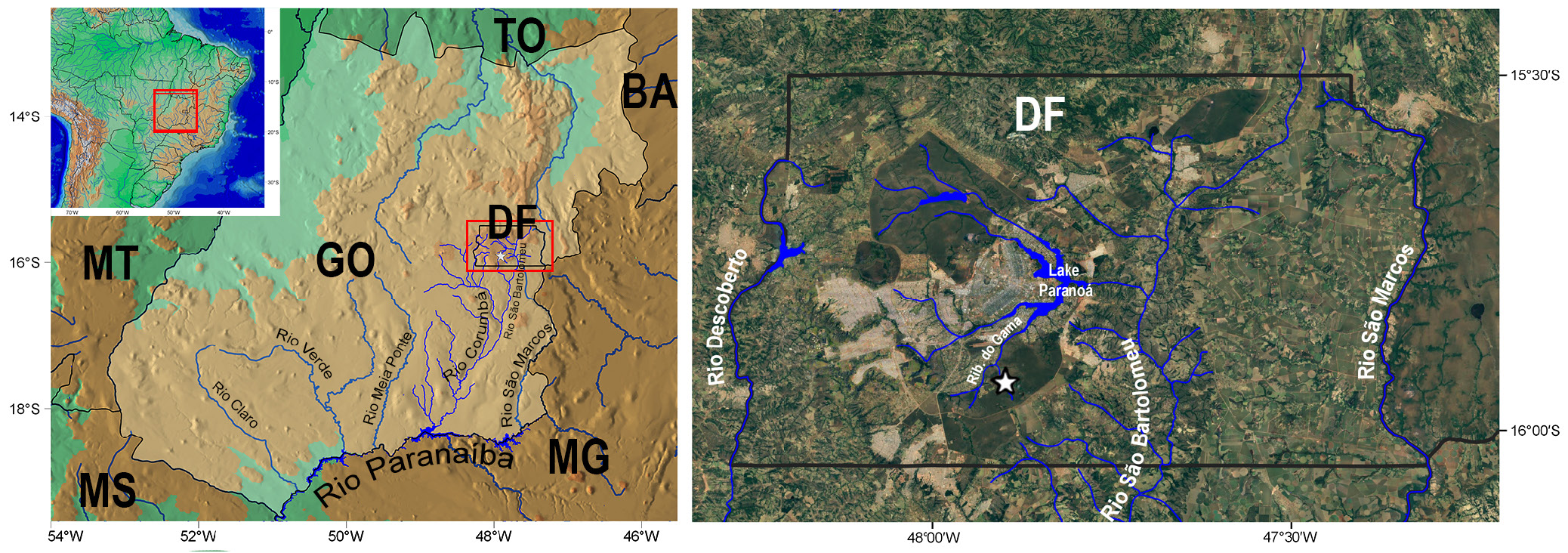
Holotype. MZUSP 125807 View Materials , 40.1 mm SL, Brazil, Distrito Federal, Brasília, córrego Taquara at Reserva Ecológica do IBGE , tributary of ribeirão Gama , tributary of Lago Paranoá , tributary of the left bank of rio São Bartolomeu , upper rio Paraná basin, 15°54’55.04”S 47°54’23.87”W, 14 Nov 2016, M. R. S. Melo & M. C. L. B. Ribeiro GoogleMaps .
Paratypes. All from Brazil, Brasília, Distrito Federal, rio Taquara , tributary of ribeirão do Gama , tributary of Lago Paranoá , tributary of right bank of rio São Bartolomeu. MNRJ 52124 View Materials , 2 View Materials , 27.1–28.4 mm SL, córrego Taquara, 15°56’29”S 47°53’52”W, 19 Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125795 View Materials (ex–IBGE 1175), 4, 28.6–30.6 mm SL, córrego Roncador , 15°56’08”S 47°53’18”W, 26 Mar 1990, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125796 View Materials (ex–IBGE1180), 3, 28.3–34.6 mm SL, córrego Taquara , 27 Mar 1990, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125797 View Materials , 2, 39.0– 44.1 mm SL, córrego Roncador , Reserva Ecológica do IBGE , 15°56’23”S 47°53’27”W, Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125798 View Materials , 7, 22.1–39.1 mm SL, córrego Taquara , 15°55’09”S 47°54’13”W, 10 Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125799 View Materials , 1, 30.4 mm SL, córrego da Onça , 15°56’25”S 47°54’05”W, 18 Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125800 View Materials , 4, 23.2–25.7 mm SL, córrego Taquara , 15°55’09”S 47°54’13”W, 19 Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125801 View Materials , 3 (1 cs), 28.8–32.8 mm SL, córrego Taquara , 15°54’36”S 47°54’30”W, 20 Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125802 View Materials , 1, 30.6 mm SL, córrego Taquara , 15°54’32”S 47°54’45”W, 20 Oct 2005, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125803 View Materials , 8 (2 cs), 28.4–41.3 mm SL, córrego Roncador , 24 Oct 2009, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125804 View Materials , 1, 36.7 mm SL, córrego Roncador at Ponte do Corujão , 15°56’07”S 47°53’29”W, 23 Sep 2009, M. C. L. B. Ribeiro; GoogleMaps MZUSP 125808 View Materials , 1, 35.4 mm SL, collected with holotype; GoogleMaps ZUEC 17241 View Materials , 4, 27.0– 34.7 mm SL, collected with MZUSP 125803 GoogleMaps ; ZUEC 17242 View Materials , 2, 38.7–40.1 mm SL, collected with MZUSP 125797 GoogleMaps ; ZUEC 17243 View Materials , 3, 23.5–26.8 mm SL, collected with MZUSP 125800 GoogleMaps ; ZUEC 17244 View Materials , 1, 31.2 mm SL, collected with MZUSP 125801 GoogleMaps .
Non-types. All from Brazil, Brasília, Distrito Federal, upper rio Paraná basin, rio Taquara drainage. IBGE 1022, 2, 31.9–36.6 mm SL, córrego Roncador; IBGE 1023, 1, 20.4 mm SL, collected with IBGE 1022; IBGE 1070, 1, 24.2 mm SL, córrego Taquara; IBGE 1089, 1, 25.4 mm SL, córrego Onça; IBGE 12, 1, 39.8 mm SL, córrego Roncador; IBGE 152, 1, 23.5 mm SL, córrego Taquara; IBGE 170, 1, 31.1 mm SL, córrego Taquara; IBGE 899, 1, 28.3 mm SL, córrego Taquara; IBGE 934, 1, 35.9 mm SL, córrego da Onça; IBGE 935, 1, 17.8 mm SL, collected with IBGE 934; IBGE 936, 1, 21.9 mm SL, collected with IBGE 934; IBGE 988, 1, 22.5 mm SL, córrego Tapera; IBGE 1729, 1, 35.4 mm SL, córrego Taquara.
Diagnosis. Characidium onca can be distinguished from its congeners except C. chancoense Agudelo-Zamora, Ortega-Lara & Taphorn, 2020 , C. japuhybense Travassos, 1949 , C. lauroi Travassos, 1949 , C. oiticicai Travassos, 1967 , C. pellucidum Eigenmann, 1909 , C. phoxocephalum Eigenmann, 1912 , C. pteroides Eigenmann, 1909 , C. schubarti Travassos, 1955 , C. stigmosum , and C. travassosi Melo, Buckup & Oyakawa, 2016 by small dots on the side of body present (vs. spots absent), and from these exceptions by the dots arranged in two or three well-marked, longitudinal lines (vs. dots irregularly distributed); and by the pectoral fin short, not reaching the vertical that passes through the origin of the dorsal fin (vs. tip of pectoral fin reaching or extending posterior to the vertical that passes through the origin of dorsal fin). It further differs from C. chancoense , C. japuhybense , C. lauroi , C. helmeri , C. pellucidum , C. phoxocephalum , C. pteroides , and C. schubarti by the adipose fin absent (vs. adipose fin present); from C. stigmosum , C. japuhybense , C. longum , and C. pteroides by the bars absent (vs. bars present); from C. japuhybense, C. lauroi , C. helmeri , C. oiticicai , C. schubarti , and C. travassosi by the isthmus scaled (vs. isthmus scaleless); and from C. pellucidum and C. pteroides by the blotches on dorsum absent (vs. sickle-shaped, reddish-brown blotches present, formed by the fragmentation of vertical bars on sides of body present), and longitudinal stripe present (vs. absent).
Description. Morphometric data summarized in Tab. 1 View TABLE 1 . Largest examined specimen 40.1 mm SL. Body elongate. Dorsal profile moderately convex between snout tip and dorsal-fin base, gently convex between dorsal and caudal-fin. Ventral profile gently convex between dentary tip and anal-fin origin, slightly convex at anal-fin base; almost straight between anal and caudal-fin bases. Belly strongly accentuated arched in females with well-developed ovarium. Greatest depth of body at dorsal-fin origin.
Snout in lateral view triangular and short, its tip at level of center of eye. Mouth small, terminal. Snout-maxillary tip about equal to diameter of orbit; tip of maxilla reaching level of anterior margin of orbit. Orbit nearly rounded, margin of orbit free. Cheek narrow, its depth less than one third of eye diameter. Nares distinctly separated, distance between nares shorter than distance between posterior naris and eye. Dermal flap along posterior border of anterior naris, and to anterior margin of posterior naris.
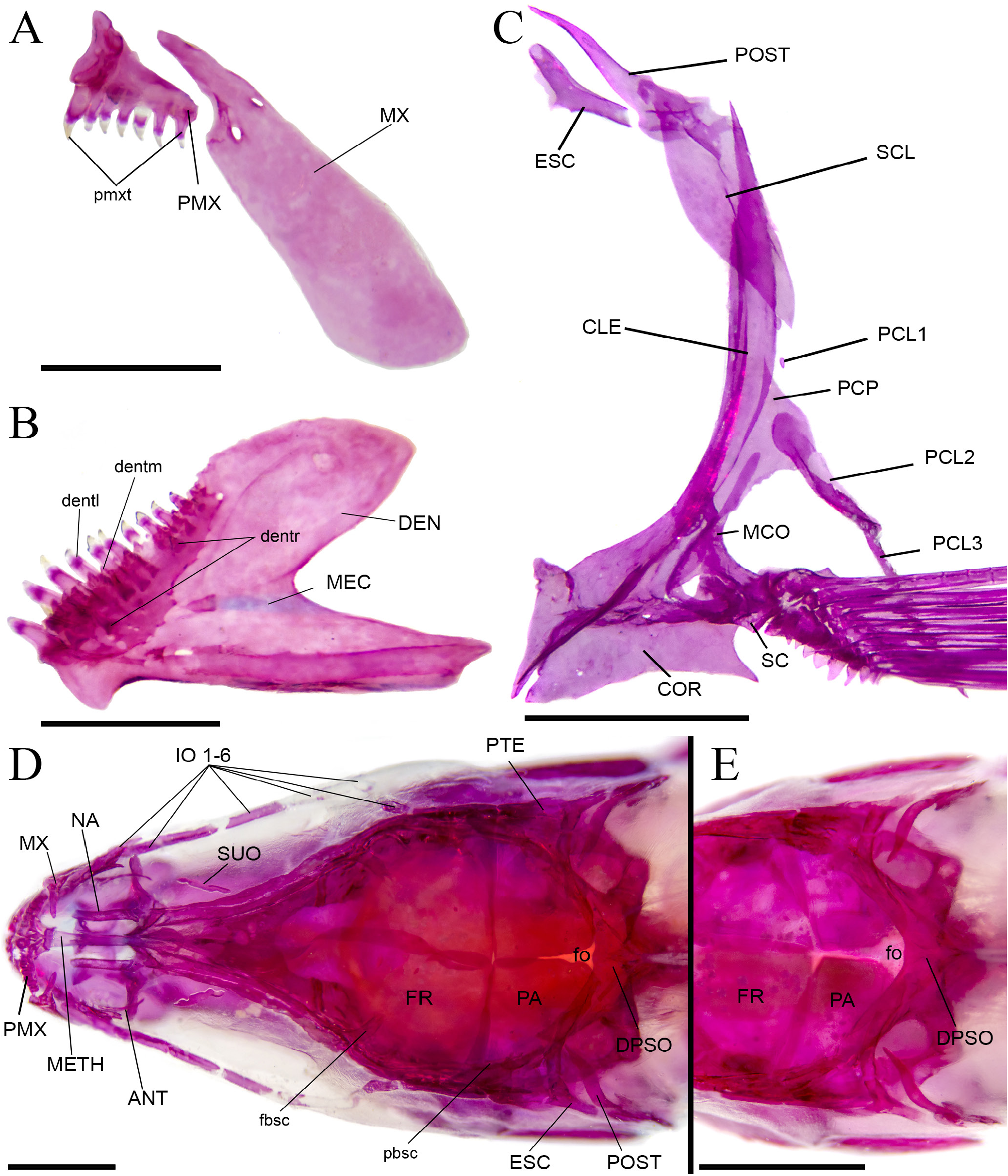
Premaxillary teeth arranged in single row, with 5(2), 6*(14), 7(7), or 8(1) unicuspid teeth, increasing in size from lateral to medial ( Fig. 3A View FIGURE 3 ). Maxillary teeth absent ( Fig. 3A View FIGURE 3 ). Dentary teeth arranged in two rows; outer row with 7(1), 6(3), 9*(16), 10(3), or 11(1) teeth, increasing in size from lateral to medial, medial teeth tricuspid with lateral cusps reduced, lateral teeth unicuspid, medial teeth larger and tricuspid; inner row with 9(1 cs), or 14(2 cs) teeth, minute and conical ( Fig. 3B View FIGURE 3 ). Ectopterygoid teeth arranged in single row, with 6(1 cs), or 7(2 cs) teeth, minute and conical. Mesopterygoid teeth absent. Branchiostegal rays 5(3 cs); four attached to anterior ceratohyal (3 cs), one attached to posterior ceratohyal (3 cs). Total gill rakers on first arch 12(2); gill rakers attached to epibranchial 4(1), or 5(2); gill rakers attached to ceratobranchial 6(1), or 7(2); gill rakers attached to basibranchial 2(3).
Scales cycloid; parallel radii present on posterior field of scale, circuli on exposed field absent. Lateral line complete, with 33(4), 34*(10), 35(8), 36(1) perforated scales. Scales above lateral line 4.5*(24). Scales below lateral line 4*(24). Circumpeduncular scales 14*(24). Pre-dorsal scales series regularly arranged; scales on pre-dorsal series 11(4), 12(14), or 13*(8). Scales between anus and anal fin 2(9) or 3(15). Isthmus scaled.
Pectoral fin short, not reaching vertical that pass through dorsal-fin origin; origin of dorsal fin at level slight anterior to pelvic-fin origin; pectoral-fin rays iii,4,iii(1), iii,5,ii*(7), iii,6,i(7), iii,6,ii(8), or iii,6,iii(1). Pelvic fin short, not reaching anus; pelvic-fin rays ii,4,i(1), i,6,i*(21), ii,6,ii(1), or i,7,i(1). Dorsal-fin rays iii,8(3), ii,9*(18), iii,9(1), or i,10(2); supranumerary element on first pterygiophore of dorsal fin 1(3 cs); last dorsal-fin ray not adnate (3 cs). Anal fin not reaching ventralmost caudal-fin ray; anal-fin rays ii,6*(24); supranumerary element on first pterygiophore of anal fin 1(3 cs); last anal-fin ray not adnate (3 cs). Principal caudal-fin rays i,8,9,i*(24); lower procurrent rays 7(2 cs), or 9(1 cs); upper procurrent rays 7(1 cs) or 8(2 cs). Adipose fin absent (24).
Precaudal vertebrae 20(1 cs), or 21(2 cs); total vertebrae 35(1 cs), or 36(2cs). Supraneurals 5(1 cs), or 7(2 cs). Postcleithrum 1 rudimentary (1) or absent (2); postcleithrum 2 and 3 present and well developed (3) ( Fig. 3C View FIGURE 3 ). Epurals 3(3 cs); hypurals 4(1 cs) or 6(2 cs). Parietal branch of supraorbital laterosensory canal present (3 cs), elongate, reaching the parietal bone. Epiphysial branch of the supraorbital laterosensory canal present. Fontanel limited antero-laterally by parietals and posteriorly by supraoccipital (2 cs) ( Fig. 3D View FIGURE 3 ), or extending anteriorly and contacting frontals (1 cs) ( Fig. 3E View FIGURE 3 ). Posterior chamber of swim bladder larger than anterior chamber, 21.9–35.3% in SL (3 cs).
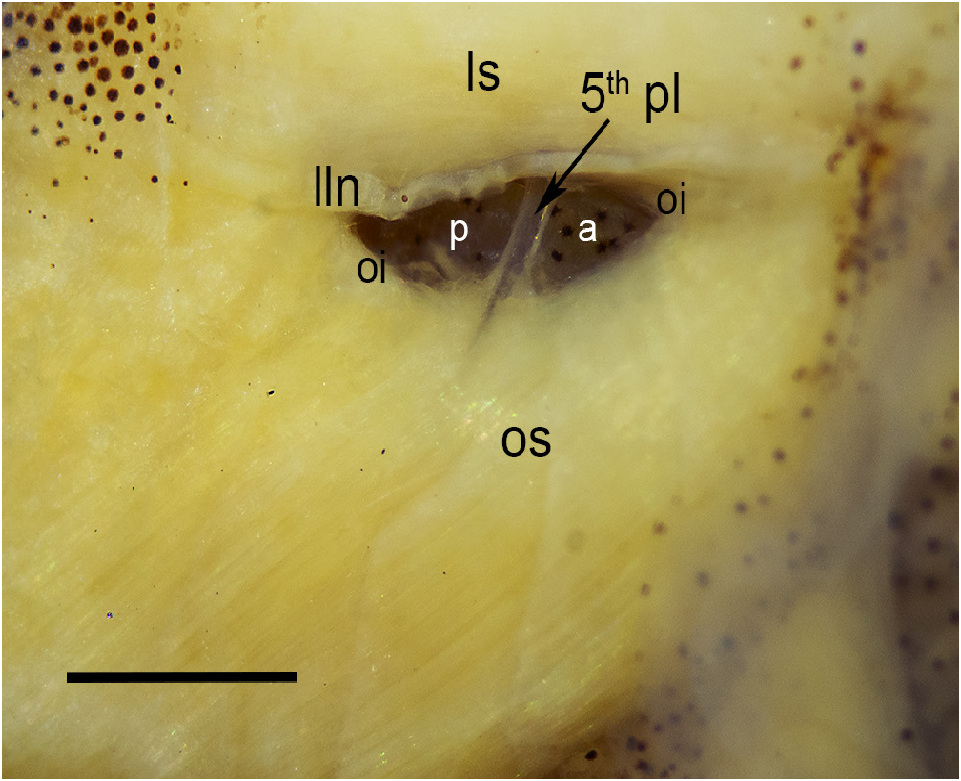
Pseudotympanum immediately posterior to supracleithrum, underneath anterior part of longitudinal stripe, antero-dorsally elongate, limited dorsally by lateralis superficialis, anteriorly and posteriorly, by obliquus inferioris, and ventrally by obliquus superioris; lined dorsally by lateral-line nerve. Humeral hiatus divided into anterior and posterior chambers by pleural rib of fifth. Fatty tissue filling humeral hiatus absent ( Fig. 4 View FIGURE 4 ).
Coloration in alcohol. Ground color of head and trunk light yellow, darker on dorsum, lighter on belly ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). Cheek, distal portion of upper jaw, ventral part of head and opercle pale with widely spaced melanophores. Dorsal portion of snout and head light brown. Oblique preorbital stripe extending from snout tip to anterior portion of eye present and conspicuous. Postorbital stripe present, conspicuous. Bars on body absent. Three thin stripes present on dorsal part of body; dorsal stripe extending from head to dorsal-fin base; middle bar extending from head to caudal peduncle; and ventral bar extending from head to base do dorsa-caudal fin ray. Midlateral stripe, stout, extending from supracleithrum to caudal peduncle, not reaching base of middle caudal-fin rays. Humeral blotch absent. Basicaudal spot inconspicuous, vertically elongated. Dots arranged in two or three longitudinal rows; dorsal row of dots extending from level of cleithrum to caudal peduncle, slightly dorsal to, or overlapped by, midlateral stripe; middle and ventral rows of dots extending from posterior angle of opercle to caudal peduncle, ventrally to lateral line. Pectoral, pelvic, dorsal, anal and caudal fins mostly hyaline, or caudal and pectoral fins dusky; melanophores present only on lepidotrichia.
Coloration in life. Overall color pattern same as preserved. Ground color yellow to brownish with dorsum darker and belly whitish, with gold tint on opercle, eye and parts of head. A longitudinal, gold stripe present, extending along body immediately dorsal to longitudinal stripe.
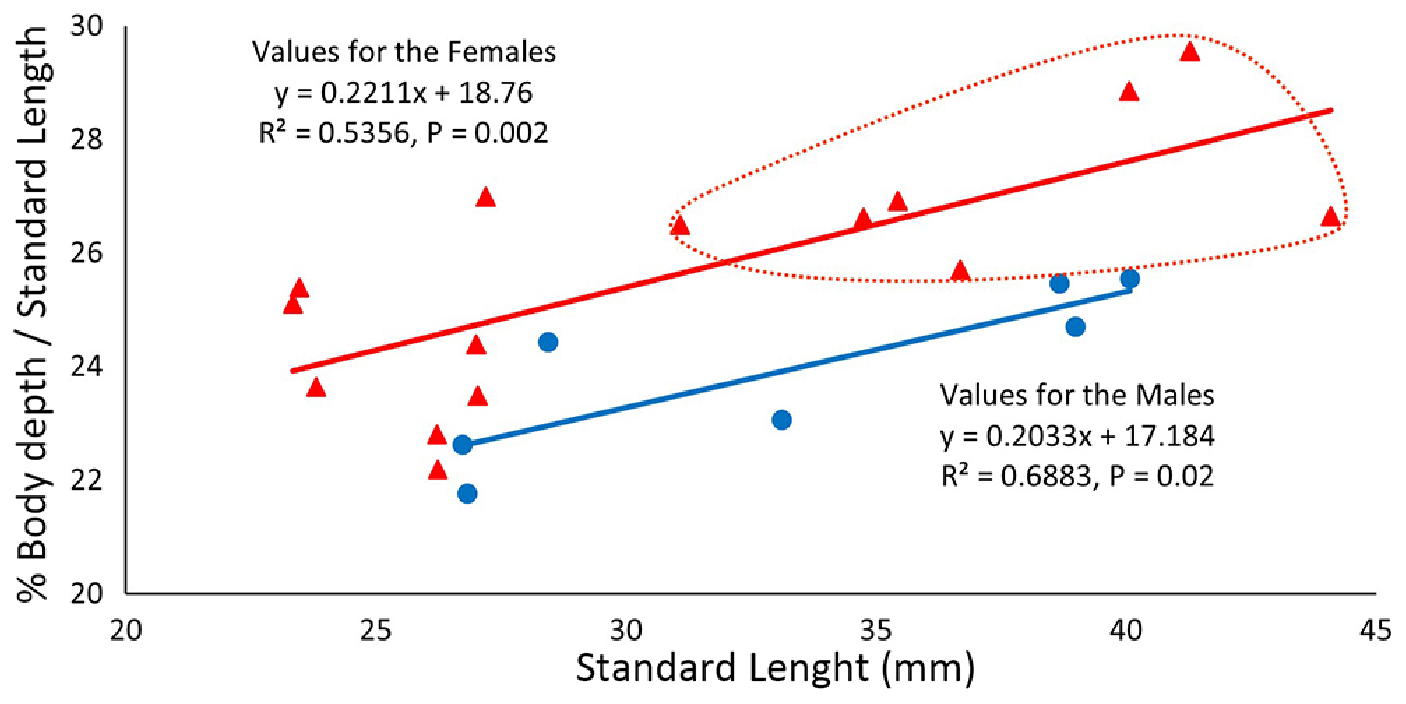
Sexual dimorphism. A total of 22 specimens had the sex and gonadal development stage evaluated, including eight immature females (23.3–27.2 mm SL), seven mature females (31.1–44.1 mm SL), and seven males (26.7–40.1 mm SL). The body depth at origin of dorsal fin has a statistically significant, positive growth in both females and males, varying from 22.2 to 27.0% in SL (mean 24.3%) in immature females; from 26.5 to 29.6% in SL (mean 27.3%) in mature females; and from 21.8 to 25.5 % in SL (mean 23.4%) in mature males ( Fig. 5 View FIGURE 5 ). The mature females with fully developed eggs have a largely expanded belly, resulting on a considerable deeper body and high standard deviation for the body depth at origin of dorsal ( Tab. 1 View TABLE 1 ). Further secondary sexual dimorphic characters, such as differences in the color pattern or the lepidotrichia bony process, are absent.
Geographical distribution. Characidium onca is only known from córrego Taquara and its tributaries, itself being a tributary of the ribeirão do Gama which nowadays drains into the lago Paranoá, a tributary of the right margin of the rio São Bartolomeu, tributary of the left margin of rio the Corumbá, rio Paranaíba drainage, in the upper rio Paraná basin, Distrito Federal, Brazil ( Fig. 6 View FIGURE 6 ).
Etymology. The specific name onca refers to the Portuguese name onça, used for the jaguar Panthera onca (Linnaeus) ( Mammalia: Felidae ), in an allusion to the black spots in a bright gold-yellow body. It is derived from the Latin lyncea, meaning lynx (Italian “lonza”, old French “l’once”), and should be pronounced as ˈõ.sɐ. A noun in apposition.
Conservation status. Characidium onca is known only from córrego Taquara and its tributaries, which have about 6.5 km of extension. Since the 1980’s, the second author ( MCLBR) has been sampling the drainages in the Distrito Federal and surroundings building a collection of over 1,000 lots of Characidium hosted mainly at the IBGE– RECOR, MZUSP and ZUEC fish collections, but C. onca was never found in any other drainages. Additional ichthyofaunistic inventories conducted in the Distrito Federal also did not report the presence of C. onca elsewhere, supporting the evidence that it truly has a narrow distribution ( Ribeiro et al., 2001, 2008; Aquino et al., 2009; Ribeiro, 2012). The extent of occurrence of C. onca is calculated in 15 km 2 and considered a single location. Additionally, the species is naturally rare and have extremely low abundance in nature ( MRSM and MCLBR, pers. obs.).
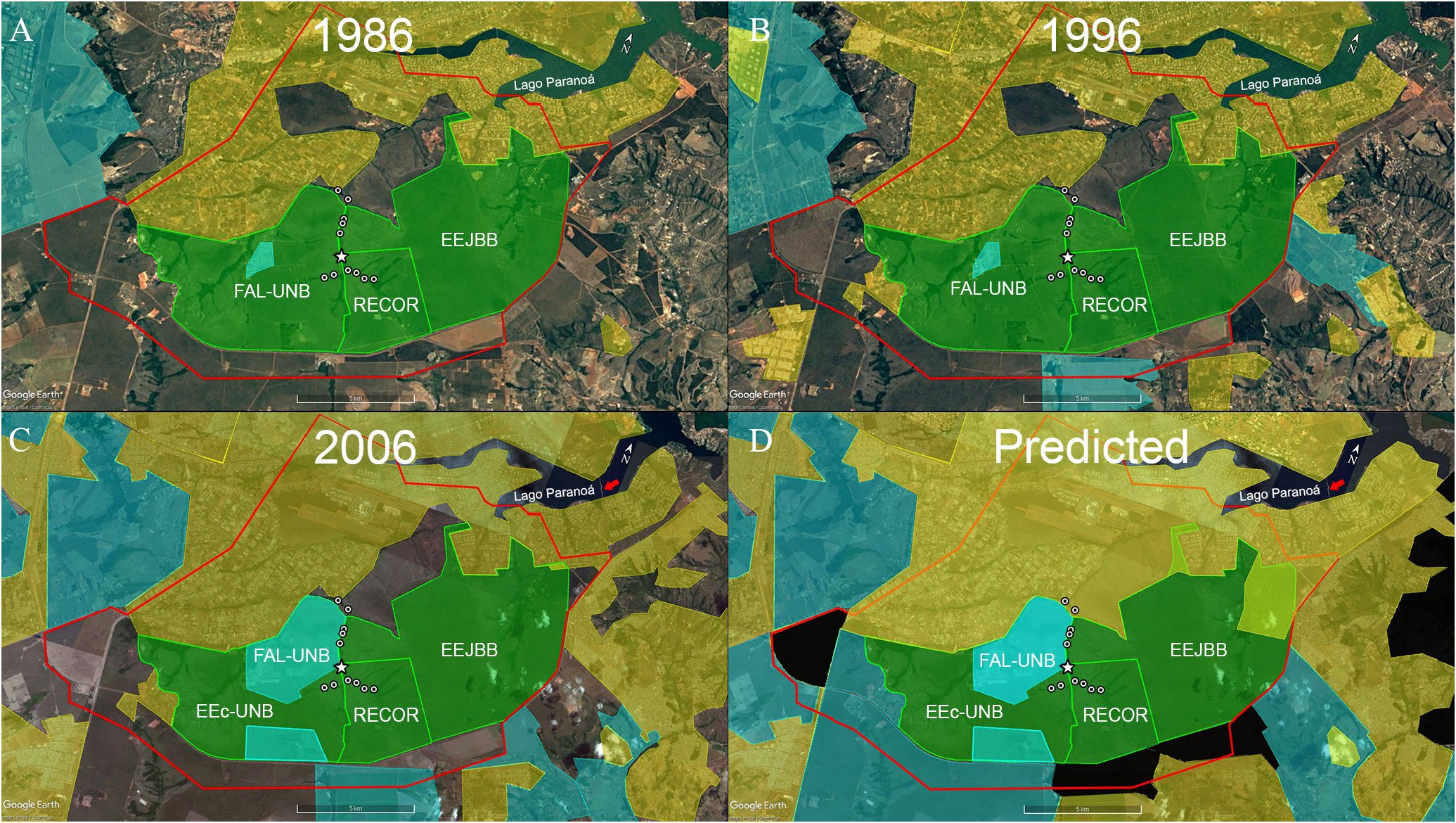
Since the construction of Brasilia in 1960’s, the impacts on the ribeirão do Gama drainage are gradually increasing ( Figs. 7A–D View FIGURE 7 ). The lago Paranoá is an artificial lake formed by damming the rio Paranoá in 1959, to provide water supply, electric power and to increase the air humidity in Brasília. The Lago Paranoá extends to the lower part of the ribeirão do Gama, slightly downstream (> 8 km) from the collection sites of C. onca , resulting on habitat loss by changing the original characteristics of a lotic into a lentic system, and by the destruction of the original riparian vegetation. The formation of the lake was followed by the introduction of several exotic species, such as the Congo tilapia Coptodon rendalli (Boulenger, 1897) , the largemouth bass (known in Brazil as black bass) Micropterus salmoides (Lacepède, 1802) , the bluegill Lepomis macrochirus Rafinesque, 1819 and the carp Cyprinus carpio Linnaeus, 1758 ( Ribeiro et al., 2001).
The construction of Brasilia also resulted in dynamic land use and vegetation coverage changes, with the replacement of natural areas of open fields (campo nativo) and Cerrado vegetation for agriculture and urban uses ( Figs. 7A–D View FIGURE 7 ). Over the last two decades, the population in the Distrito Federal increased in more than 70%, from 1,737,813 in 1995 to 2,977,216, in 2016 ( UNESCO, 2002). The rapid urbanization has replaced large areas of the natural Cerrado ecosystem with urban areas and agriculture land for cultivating soybean, corn, and bean. The intense use of surface and underground hydrological resources for such purposes caused water depletion and local climate changes of longer dry seasons and a predicted increase of temperature by up to 5 o C ( Campos, 2004; Cadamuro, Campos, 2005; Lorz et al., 2012; IBGE, 2017). The most severe consequences of those impacts are loss of ecological integrity of both terrestrial and aquatic ecosystems, such as the reduction of water level in lakes, rivers and floodplains, the deterioration of water quality, changes in the fire regimes, loss of gallery forests, and the increase of river channel sedimentation that reduces habitats conditions for the species of Characidium ( Alley et al., 1999; Ribeiro et al., 2018).
The distribution of C. onca is within the Area de Proteção Ambiental ( APA) das Bacias do Gama e Cabeça de Veado, created by the Decreto Distrital Nº 9.471, of April 21 st, 1986 and protected by both district (Reserva da Biosfera do Cerrado, Lei Distrital Nº 742, of July 28 th, 1994) and federal laws ( ARIE dos córregos Capetinha e Taquara, Decreto Federal Nº 91.303, of June 3 rd, 1985; APA do Planalto Central, Decreto s/nº da Presidência da República, of January 10 th, 2002). The APA das Bacias do Gama e Cabeça de Veado is composed by a relatively large mosaic with more than 10,000 hectares of protected areas, including the Reserva Ecológica do IBGE ( RECOR), Estação Experimental Fazenda Águas Limpas ( FAL – UNB) of the University of Brasília and Estação Ecológica do Jardim Botânico ( EEJBB) de Brasília, among others. The ARIE dos córregos Capetinha e Taquara was initially classified as a “Zona de Conservação Ambiental” and had a relatively low protection stability ( UNESCO, 2003) ( Fig. 7 View FIGURE 7 ).
Because of its proximity to downtown Brasília, a considerable extension of the APA das Bacias do Gama e Cabeça de Veado is already occupied by urban areas, especially in the ribeirão do Gama left margin. The mosaic composed of the FAL – UNB, RECOR and EEJBB is also under strong agricultural and real state speculations and the plans to expand the city limits includes the construction of the Juscelino Kubitschek bridge in 2002 and several changes in delimitation and management categories of the protected areas occasioned by the revision of the Distrito Federal Master Plan (Plano Diretor de Ordenamento Territorial – PDOT, Lei Complementar Nº 17/1997, of January 23 rd, 1997, revised by the Lei Complementar Nº 803/2009, of April 25 th, 2009, and instituted by the Lei Distrital No 6.269, of January 29 th, 2019). The FAL – UNB, was replaced with the Estação Experimenal da UNB and, within that area, the Estação Ecológica da UNB (EEc– UNB) was circumscribed as a “Macrozona de Proteção Integral”, the RECOR and the EEJBB were classified as a “Macrozona de Proteção Integral”, but the latter had a part of its territory changed to “Urban Zone”. The expansion of human occupation in the vicinity of the mosaic resulted on the habitat fragmentation and the loss of ecological integrity of both terrestrial and aquatic ecosystems, compromising the creation of buffer zones and ecological corridors ( UNESCO, 2003; Ribeiro et al., 2018).
In summary, C. onca has a very narrow distribution in a single location that has observed impacts of human occupation and introduction of exotic species in the basin and nearby areas, and predicted impacts of reduction of the river flow and loss of water quality caused by the longer periods of the dry season and higher temperatures due to local climate changes allied to the intense use of surface and underground hydrological resources. Following the IUCN Red List Categories and Criteria ( IUCN Standards and Petitions Subcommittee, 2019), C. onca should be categorized as Critically Endangered (CR) because of its restricted geographic distribution (B1: Extent of occurrence <100 km 2) and the following two conditions: number of location equals one (a); and continuing decline observed, estimated, inferred or projected of area extent and/or quality of habitat (biii).
Comparative material examined. Same as listed in Melo et al. (2016); Melo, Espíndola (2016); Teixeira, Melo (2021).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |