Convolutriloba macropyga, Iii, Thomas Shannon & Achatz, Johannes G., 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.177524 |
|
DOI |
https://doi.org/10.5281/zenodo.6251920 |
|
persistent identifier |
https://treatment.plazi.org/id/03D43255-3A61-FFFF-FF4C-C106E40A5ABD |
|
treatment provided by |
Plazi |
|
scientific name |
Convolutriloba macropyga |
| status |
sp. nov. |
Convolutriloba macropyga sp. nov.
Diagnosis. Convolutriloba with sparsely but widely distributed concrements on the dorsal surface; one type of rhabdoid gland cell with 3-µm long rhabdoids. Male copulatory organ consists of paired, lateral, sclerotized canals leading into a seminal vesicle. Seminal vesicle opens into a vesicula granulorum, which is filled with prostate secretion in its proximal part and cyanophilic vesicles in its distal part. Animals have 1 to 3 bursal nozzles. The mouth is positioned at 31 U of total body length (percent, measured from anterior tip to edge of posterior lobe); the female gonopore is at 52 U; the male gonopore is at 75 U.
Type material. Holotype: USNM 1100318, one complete set of 2-µm-thick serial cross sections. Paratypes: USNM 1100329, one partial set of 2-µm-thick serial sagittal sections, USNM 1100330, USNM 1100331, two partial sets of 2-µm-thick serial cross sections.
Type Repository. Smithsonian Natural History Museum, Washington D.C., USA.
Type Locality. Tropical marine aquarium at Cappuccino Bay Aquarium, Marietta, GA, USA.
Etymology. Specific epithet is a derivation of the Greek macro - (large) and pyga (rump), and reflects the extensive expansion of the posterior region of the body, especially while basking.
Other material examined. Living specimens and eggs in squeeze preparations; 12 whole-mount specimens for fluorescence microscopy; four partial serial sections stained with toluidine blue.
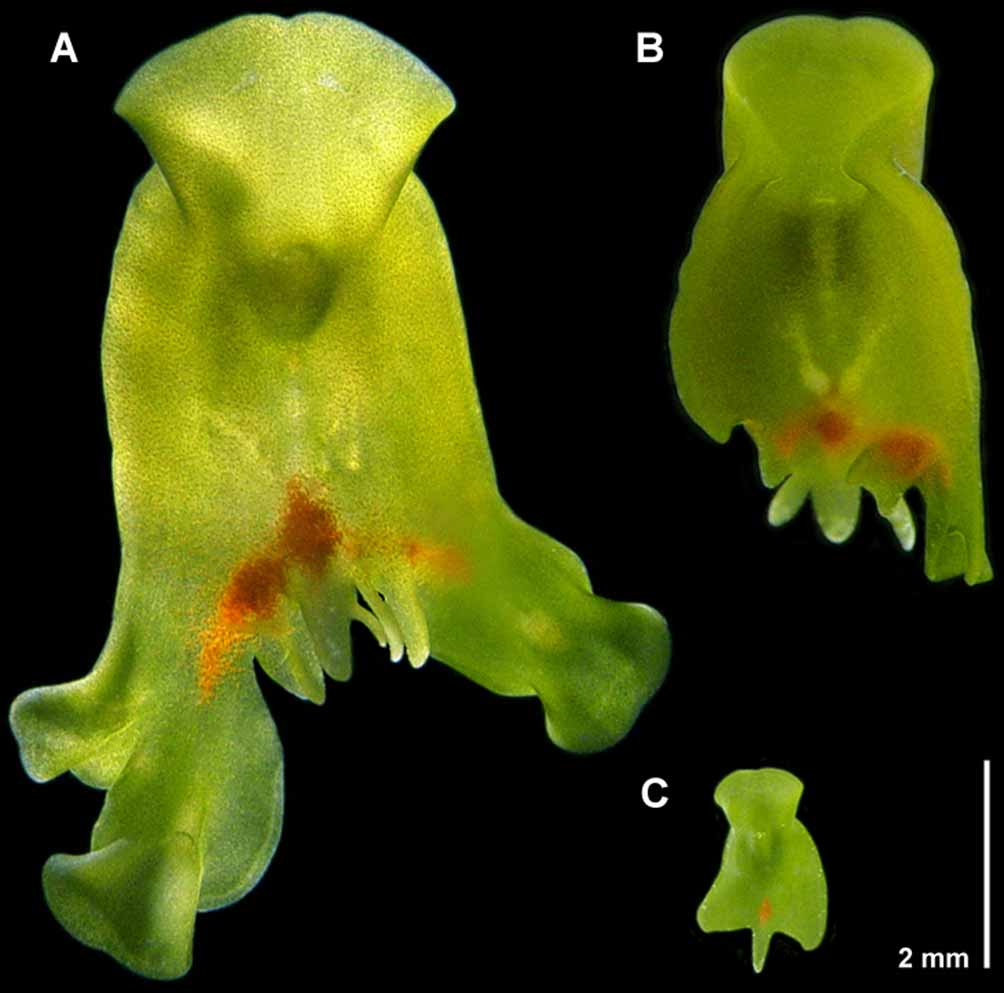
External morphology & behavior. Convolutriloba macropyga sp. nov. is flat and shield-shaped, its body rounded anteriorly and indented at the anterior tip, broadening to auricular apices set off by a transverse constriction ~ 2 mm behind the anterior tip, and broadening toward two rounded lateral caudal lobes and a longer, slender median caudal lobe ( Figs. 1 View FIGURE 1 A–C, 2A). Immature specimens always possess the three caudal lobes ( Fig. 1 View FIGURE 1 C), but adults often develop multiple median lobes ― usually 2 or 3, and up to 9 ( Fig. 1 View FIGURE 1 A). Individuals are often observed lying stationary with their anterior end erected into the water column in well-illuminated areas. When basking like this, the body is dorso-ventrally flattened to a thickness of 200–360 µm along the lateral margins and 550 µm along the median line. Mature basking specimens are up to 8 mm long and 6 mm wide.
When motile, adult specimens measure up to 10 mm in length and, apart from slight indentations in the lateral margins at the transverse constriction, are uniformly 1.5–2.5 mm wide along the entire length as the body is held tube-like, with the lateral margins curled ventrally. The animal glides by ciliary action.
A sudden increase in light intensity triggers a negatively phototactic, or photophobic, behavior. Mechanical disturbance of specimens in glass culture dishes trigger rapid, forward motion. Similar disturbances in more natural environments cause the animal to move to the shaded undersides of objects or into the substrate.
When food is present, as when Artemia sp. are added to the cultures, animals lift the anterior body tip from the substrate and curl the lateral edges and the two ventral flaps ( Fig. 2 View FIGURE 2 B) anterior to the transverse constriction ventrally, forming a “capturing funnel” ( Fig. 1 View FIGURE 1 B) sensu Hendelberg & Åkesson (1988). The funnel leads to the mouth, which is located medially on the ventral side ~ 2 mm behind the anterior tip ( Figs. 2 View FIGURE 2 B, 3A). Though some animals will move in the direction of the prey, most remain relatively motionless with their posterior lateral margins attached to the substrate. When prey moves into the funnel, the animal traps it by pressing down flat against the substrate and moving forward to bring the prey into its mouth.
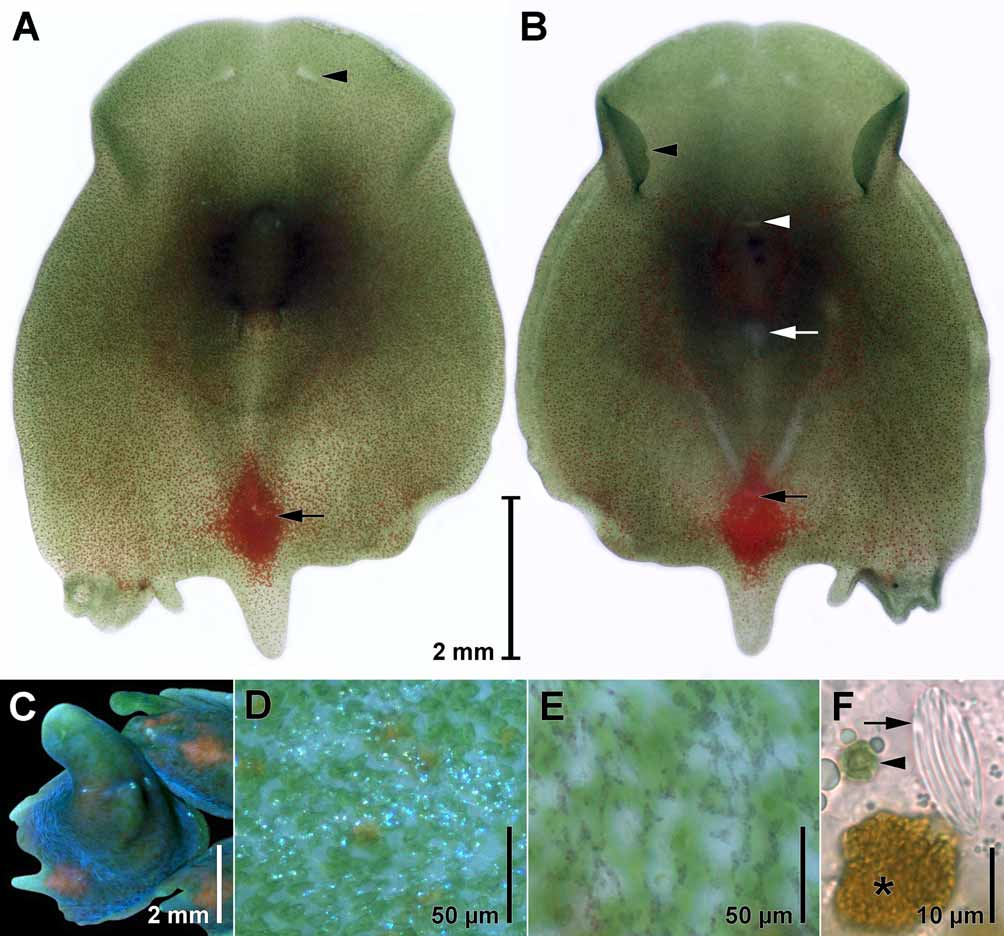
Body coloration is green, tinged with red, due to symbiotic zoochlorellae and scattered, red rhabdoid gland cells and a diamond-shaped red spot comprised of pigment cells in front of the caudal lobe. The dorsal body surface appears bluish in reflected light due to refractive concrements ( Figs. 2 View FIGURE 2 A–F).
Epidermis. The epidermis is entirely ciliated. The cilia are commonly ~8 µm long, but can measure up to 12 µm in some areas. On the ventral side of the capturing funnel, the cilia are often sparse or shorter. The epidermal nuclei are sunken beneath the body-wall musculature. Small refractive epidermal concrements occur in large fields. The density of these fields increases gradually from the anterior to the posterior end. The concrements give the surface a bluish sheen under incident light and appear dark purple in transmitted light ( Figs. 2 View FIGURE 2 C–E). The sheen vanishes when animals are relaxed in magnesium sulfate. Dorsally at the transverse constriction, three to five small white spots of concrements occur in a transverse row. Similar spots occur along the lateral margins in some individuals.
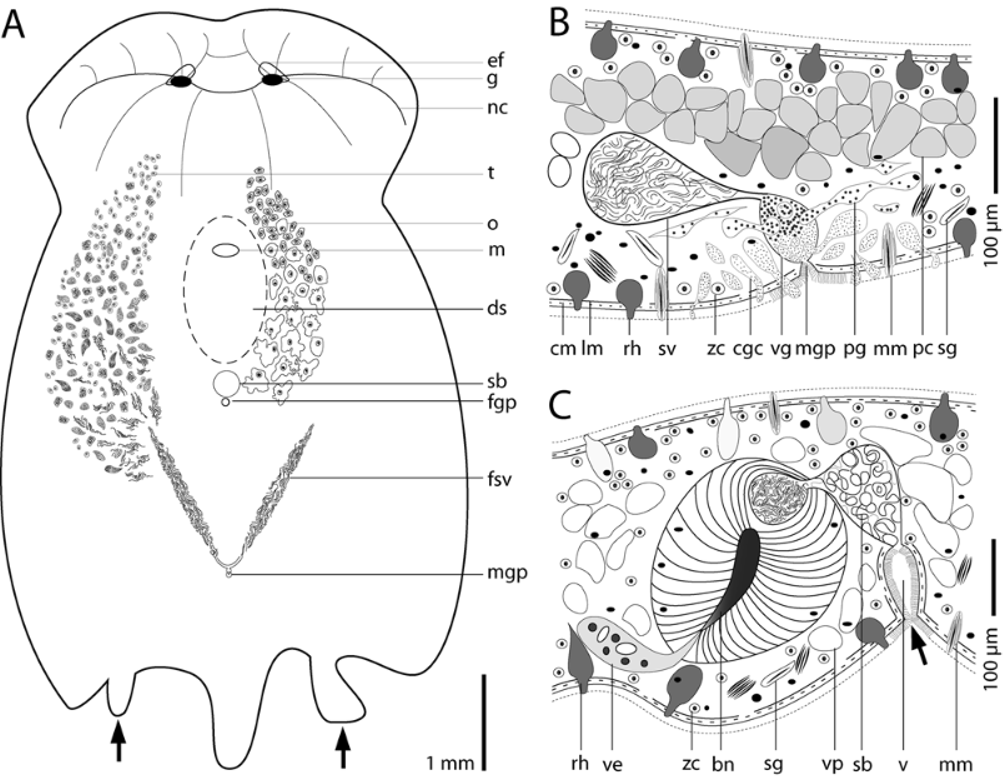
Sensory organs & nervous system. A pair of eye fields ( Fig. 2 View FIGURE 2 A) appearing colorless due to the absence of symbiotic algae occurs ~650 µm behind the anterior tip. Paired, insunk ganglia lie ventral to them. The ganglia are connected transversally by a commissure. From each ganglion, two nerve cords run frontally, one runs laterally and one runs latero-caudally. A pair of median longitudinal nerve cords originates at the commissure ( Fig. 3 View FIGURE 3 A) and can sometimes be seen in live animals as two colorless stripes due to the absence of zoochlorellae. A statocyst is absent in all adult specimens examined, but present in juveniles ( Fig. 7 View FIGURE 7 A).
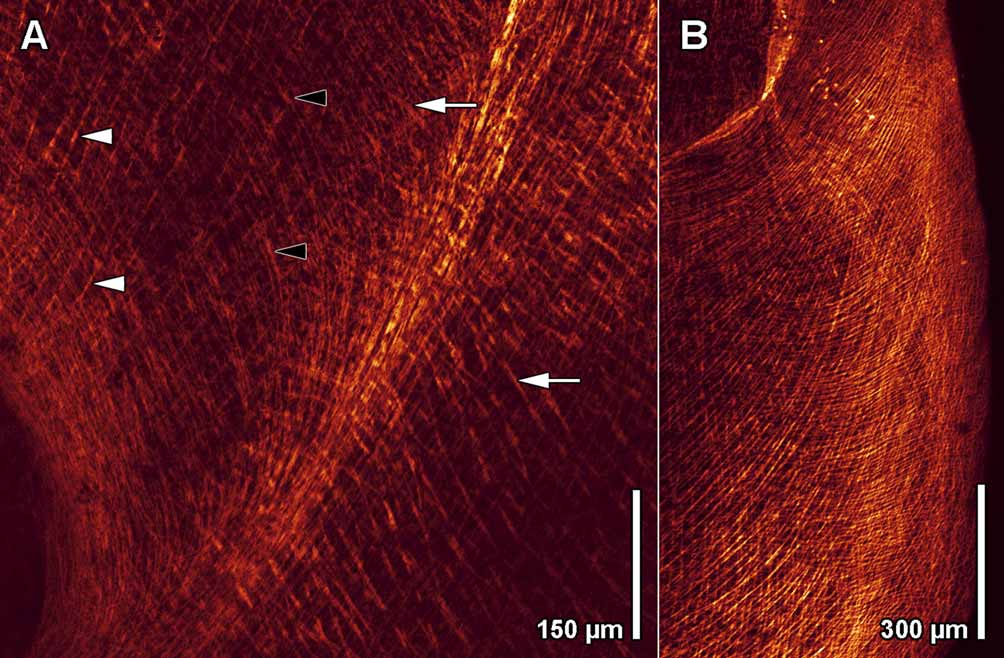
Musculature. The body-wall musculature is stronger on the ventral than on the dorsal side. The dorsal musculature consists of outermost circular, diagonal, and longitudinal muscles. The ventral musculature consists of outermost circular muscles ( Fig. 5 View FIGURE 5 B), a layer of muscles that arc across the body in curves centered on the mouth ( Fig. 5 View FIGURE 5 C), and an innermost layer of longitudinal muscles and muscles radiating from the mouth ( Fig. 5 View FIGURE 5 D). Circular muscles near the lateral posterior edge of the mouth bend around its anterior rim in a Ushaped path. Some of these do not bend fully around the mouth but run anteriorly and terminate lateral to it. The next layer inward consists of muscles surrounding the mouth and constituting the wall of the capturing funnel. They bend around the posterior rim of the mouth and run straight and oblique anteriad, crossing each other in front of the mouth ( Figs. 4 View FIGURE 4 A, 5C). In the posterior half of the body are corresponding longitudinal cross-over muscles ( Figs. 4 View FIGURE 4 B, 5C). The innermost layer consists of special pore muscles, which fan out from the mouth to the anterior rim and the lateral edges of the capturing funnel ( Figs. 4 View FIGURE 4 A, 5D), and longitudinal muscles at the posterior end ( Figs. 4 View FIGURE 4 B, 5D). These longitudinal muscles insert slightly in front of the posterior rim of the capturing funnel ( Fig. 4 View FIGURE 4 A). Dorso-ventral muscles are abundant, especially laterally.
Gland cells. Numerous adhesive papillae are distributed along the posterior lateral margin. They comprise the distal tips of glands protruding through the body wall and are ~5 µm long and 2 µm wide.
Two sorts of rhabdoid gland cells, whose cell bodies lie in the parenchyma, protrude on the body surface. The cells of the first type are highly flexible in shape but are commonly ~45 µm long and ~15 µm wide and contain ~250 rhabdoids measuring 2–3 µm long and ~1 µm wide, the contents of which are reddish-orange ( Fig. 2 View FIGURE 2 F). Some of these rhabdoid gland cells bear similarly shaped translucent rhabdoids instead. The rhabdoids and the cytoplasm of these cells are cyanophilic. The cells are distributed on the dorsal and ventral side, with the exception of the ventral side of the capturing funnel, including the inner side of the ventral flaps. In non-sexual juvenile specimens the red rhabdoid gland cells are sparsely distributed. As an animal matures, cell densities increase body-wide with higher densities emerging both dorsally and ventrally adjacent to the developing ovaries. In sexually mature adults rhabdoid gland cells are highly numerous along the posterior, lateral margins, the lobes, around the male copulatory organ, and in the region of the gonads on the ventral side ( Figs. 2 View FIGURE 2 A, B). The second rhabdoid gland cell type occurs solely on the dorsal side, about 20 cells in a specimen. Each cell contains ~18 refractive rods, which are ~20 µm long, 1 µm wide ( Fig. 2 View FIGURE 2 F), and strongly cyanophilic.
Mucous gland cells are absent. Adults lack a frontal organ, but freshly hatched juveniles have an easily recognized frontal organ with a reservoir, all lying in front of the statocyst ( Figs. 7 View FIGURE 7 A, B).
Red pigment cells are densely packed on the dorsal side in a diamond-shaped red spot ~ 1.4 mm long and ~ 0.9 mm wide in front of the median caudal lobe ( Figs. 1 View FIGURE 1 C, 2A, B). The cells lie dorsal to the male copulatory organ and ventral to the body-wall musculature and the rhabdoid gland cells, they do not protrude to the surface, and measure 40–50 µm in diameter ( Fig. 3 View FIGURE 3 B). In histological sections the cells are filled with a grayish meshwork and their cytoplasm is not stained.
Sagittocysts occur in two sizes. Large sagittocysts occur in abundance and measure ~40 µm long and ~2.5 µm wide. Small sagittocysts, distributed mainly at the anterior lateral margins, measure ~20 µm long and ~1 µm wide. The sagittocysts are formed in sagittocytes which lie ventral to the dorsal body-wall musculature and rhabdoid gland cells. The sagittocytes generally contain a bundle of 8–12 sagittocysts. Each sagittocyst moves towards the distal tip of the sagittocyte, which lies within the body wall. A muscle cell, or muscle mantle, enwraps the sagittocyst within the distal tip of the sagittocyte. This arrangement is connected with a sensory cell and altogether is called an extrusion apparatus sensu Gschwentner et al. (2002). The muscle mantles enwrapping the small sagittocysts are ~50 µm long and ~6 µm wide, those enwrapping the large sagittocysts are ~75 µm long and ~9 µm wide. The extrusion apparatus are distributed over the entire dorsal surface and the lateral sides of the ventral flaps, with higher densities found on the lateral margins. Ventral distribution is limited to the area between the female gonopore and the caudal end with high densities in a broad region between the gonopores.
Parenchyma & zoochlorellae. The parenchyma consists of dense peripheral parenchyma and parenchyma cells with large vacuolated spaces. The dense parenchyma occurs primarily in the periphery of the body, but is also found centrally surrounding nervous tissue and often forms extensions into the vacuolated parenchyma. Numerous zoochlorellae, 5–14 µm wide, are distributed throughout the parenchyma. In squeeze preparations and motile specimens, zoochlorellae appear to be arranged in rows, mirroring the overlying musculature (and sometimes the longitudinal nerves, as well); a random distribution is observed in specimens at rest. The algal endosymbiont has been isolated using the CO2 bubbling method of Boyle & Smith (1975) and cultured in L1 media. We have not yet identified the algal species.
Testes & male reproductive system. The paired testes lie dorsal and lateral to the ovaries. Follicles and sperm pass caudally and sperm accumulate in paired false seminal vesicles, which measure ~170 µm in diameter and converge toward the body-midline ( Fig. 3 View FIGURE 3 A). Mature spermatozoa measure ~280 µm long, have a thin ~50 µm long tail, and a stepped, ~20 µm long tip.
The male gonopore lies about 1 mm in front of the posterior end, slightly less than 2 mm behind the female gonopore, and opens into a vesicula granulorum. The vesicula granulorum, ~50 µm in diameter and ~75 µm high, lies within a plug of peripheral parenchyma, which is 450 µm long, 300 µm wide, and 150 µm high. Dorso-ventral muscles, sagittocytes, extrusion apparatus, and two types of gland cells are embedded in this plug. The first type of gland cell contains small cyanophilic vesicles with a diameter of 300–500 nm, and protrudes through the body wall around the male gonopore and into the distal part of the vesicula granulorum. The second type, prostate gland cells sensu Winsor (1990), produce basophilic vesicles with a diameter of ~1 µm, and protrude exclusively into the proximal part of the vesicula granulorum. A seminal vesicle, measuring ~100 µm in diameter when filled with sperm, opens into the proximal end of the vesicula granulorum ( Fig. 3 View FIGURE 3 B). One lateral canal on each side connects the seminal vesicle with the caudal end of the corresponding false seminal vesicle. Each canal is ~ 300 µm long and has a diameter of 22 µm. The paired canals and the seminal vesicle are surrounded by sclerotized tissue and parenchymal musculature.
Ovaries & female reproductive system. The paired ovaries lie ventral and medial to the testes ( Fig. 3 View FIGURE 3 A). Early oocytes contain numerous translucent granules, have a cell diameter of ~50 µm, a nucleus measuring ~10 µm, and a nucleolus measuring 2–5 µm in diameter. The nucleus is surrounded by dense homogeneous cytoplasm measuring 25 µm in diameter. During cellular growth the size and morphology of the nucleus and nucleolus remain constant as the cell becomes larger and lobulated. In living specimens one can observe the appearance of orange-brown granules in the cytoplasm of oocytes at about the level of the mouth. At the same level, basophilic granules with a diameter of 500–800 nm start to appear in histological sections. As oocytes mature the cytoplasm stains progressively darker.
The female gonopore lies ~ 1.5 mm behind the mouth ( Fig. 3 View FIGURE 3 A). The vagina is an invagination of the body wall ~100 µm long, ciliated, and lined with weak musculature ( Fig. 3 View FIGURE 3 C). The vagina connects with the distal part of the seminal bursa, which is lined with weakly sclerotized tissue and often filled with spongy tissue. The proximal part is surrounded by bursal nozzle tissue. Bursal nozzles range in size from 55 µm to 150 µm and vary in number from 1 to 3 ( Figs. 6 View FIGURE 6 A, B). Of 26 specimens examined, 15 had one nozzle, 7 had two, and 4 had three. All sectioned animals had two bursal nozzles lying in close proximity, sharing one common seminal bursa. The bursal nozzles are directed antero-ventrally, and curve frontally. The vestibula are extraordinarily large and contain many rounded nuclei, cyanophilic vacuoles, and a weakly stained granular plasma.
Sexual reproduction. Although we have not yet witnessed copulation, sexual reproduction is evident in our populations and sexually mature animals routinely produce eggs. One third of the adults in the progenyrelease trials laid eggs after being individually isolated at the onset of the experiment. Egg laying was most common in the first five days but continued until the ninth day of isolation. Most animals laid eggs on three or four different days; one animal produced eggs on seven consecutive days.
Eggs are commonly laid in a flat cluster measuring no more than 2 mm in diameter and are suspended in a transparent matrix that adheres the cluster to the substrate. Of fifty clusters collected for egg counts, average number of eggs per cluster was 78; the smallest cluster contained 31 eggs, and the largest contained 181. Eggs are ovoid in shape with a thin, transparent shell ~170 x 130 µm. Embryos in freshly laid eggs bear a reddishorange color, have no readily identifiable morphological features, and occupy ~90% of the egg. After 36–48 hours, a darkening, dense, red spot appears within each embryo. The embryos’ surfaces are entirely ciliated, and they rotate within the eggs. By the third day the embryos are folded over ventrally, continue to rotate, have fully developed red rhabdoid gland cells concentrated centro-caudally and laterally, and a statocyst. Juveniles begin to hatch on the third day and most have emerged by the end of the fourth. Hatchlings are ~230 µm long and ~120 µm wide, dorso-ventrally flattened, rounded anteriorly and taper caudally to a rounded point ( Fig. 7 View FIGURE 7 A). They harbor no algal symbionts, but have a frontal organ, a statocyst with a statolith, and approximately 100 red rhabdoid gland cells ( Figs. 7 View FIGURE 7 A–D). The statocyst is ~22 µm and the statolith ~12 µm wide ( Fig. 7 View FIGURE 7 C). Four-day-old hatchlings already possess small sagittocysts at the anterior end. Hatchlings glide along the substrate using their cilia; unlike adult animals they routinely swim freely in the water column by cilia. They appear to consume bacteria, as evidenced by large numbers of live bacterial cells sequestered in the central parenchyma, and have been observed preying on small ciliates.
Asexual reproduction. This species reproduces asexually by reverse budding ( Hendelberg & Åkesson 1988) whereby the main axis of the progeny is reversed 180° relative to that of the mother. Budding begins as a thickening, slight protrusion anywhere along the caudal margin lateral to the median lobe(s), up to and including the lateral lobes. Concomitant with the thickening is a migration of zoochlorellae to the protrusion rendering it a darker green, and the emergence of red pigment cells medial to, and forward of the protrusion in the mother. Within 24 hours the bud has elongated disto-caudally from the mother and the newly formed red pigment spot has expanded and elongated to span the marginal interface between mother and daughter as can be seen in Figure 1 View FIGURE 1 A. Within the following 24–36 hours a head has developed, eye fields are evident, and in some cases the daughter begins feeding holozoically. Shortly thereafter, the bud is not released but torn from the mother when it attaches itself to the substrate and pulls away. At any one time, we have observed upwards of 4–5 daughter individuals in various stages of development on a mother individual.
Released progeny size is directly proportional to the size of the mother animal. Newly released progeny ranges from 1 to 3 mm in length. Larger progeny can produce buds within 24 hours. Sexual maturity is reached within 8–10 days under optimal environmental conditions. Growing buds and newly released progeny exhibit the characteristic refractive blue sheen of their mother. Soon after release, however, the concrement densities decrease to a sparse distribution primarily in the caudal half of the juvenile animal. As the animal matures the concrement distribution expands and becomes denser such that sexually mature adults have the refractive blue sheen over the entirety of their dorsal surface.
Progeny release rates. Rates appear to vary in response to many environmental factors including, but not limited to, prey availability, diversity of prey, water quality, water flow, and light quality. Of the abiotic variables, light intensity appears to have the greatest influence on asexual budding ( Fig. 8 View FIGURE 8 A). Control animals kept in darkness released about one bud every 10 days; those in high light released one bud every 6.4 days; and those under optimal light conditions released one bud every 2.9 days.
Environmental limitations. Salinity-tolerance experiments on adult specimens revealed 50% lethality concentrations at 24 ppt and 44 ppt over a three-day exposure with an optimal salinity (100% survival) of 34 ppt (Fig. 9A). Temperature experiments showed a 100% survival range of 18–28°C. Unlike the relatively gradual decreases in survival observed in the salinity experiments, abrupt drops to zero-survival occurred immediately outside this range (Fig. 9B).
Comparative data. We found few pronounced differences among species of Convolutriloba in their sexual reproduction. Egg size was statistically larger in C. hastifera than in the three other species (180 x 125µm in C. hastifera vs. 170 x 130 µm; one-way ANOVA with length:width ratio as the independent variable, α = 0.05, p <0.001, post-hoc Tukey HSD test verified statistical difference of C. hastifera egg size), but there was no difference in embryo size. Embryos developed similarly in all species and hatched within the same 3-to-4- day window. All species’ hatchlings were aposymbiotic and possessed a frontal organ and statocyst. Aposymbiotic hatchlings maintained their size for about one week, then gradually decreased in size and died within two weeks. We do not know how any of the species obtains algal symbionts in the wild, but one-day-old hatchlings of C. macropyga were successfully infected with symbionts when algal (previously isolated from C. macropyga ) culture was added to dishes containing the hatchlings.
Our comparative dark-survival data ( Fig. 8 View FIGURE 8 B) show that Convolutriloba macropyga sp. nov., C. retrogemma , and C. longifissura , experience 100% mortality after approximately 23–26 days in total darkness with access to prey. Convolutriloba hastifera , however, survives for 8 days more. In all species, as zoochlorellae density decreased, density of red rhabdoid gland cells increased. Prior to death, all animals, regardless of species, were primarily red-orange in color and were a fraction of their original size despite having captured and consumed Artemia sp.
FIGURE 9. Environmental tolerances of Convolutriloba macropyga sp. nov.; A. Salinity tolerance. Animals were subjected to a range of artificial seawater salinities, n = 6 per salinity. Surviving numbers were recorded every 12 hours for three days. No change in survival percentages was noted after 60 hours. Second order polynomial regressions were fit to each data set; r2 = 0.81 for 60-hour regression line. Fifty percent lethality occurred at 24 and 44 ppt. B. Thermal tolerance. Animals were subjected to a range of temperatures, n = 20 at each temperature tested. Surviving numbers were recorded every 24 hours for three days.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
SubPhylum |
Acoelomorpha |
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |