Mastacembelus ophidium Günther, 1893
|
publication ID |
https://doi.org/ 10.1080/00222930400002887 |
|
publication LSID |
lsid:zoobank.org:pub:49C8DDC3-5FF2-47BC-8BD7-427B654A1122 |
|
persistent identifier |
https://treatment.plazi.org/id/6A48E935-FFF7-FFBB-3ED9-FD8B3E97F924 |
|
treatment provided by |
Felipe |
|
scientific name |
Mastacembelus ophidium Günther, 1893 |
| status |
|
Mastacembelus ophidium Günther, 1893 View in CoL
( Figure 6 View Figure 6 )
Synonyms and citations
Mastacembelus ophidium Günther, 1893 View in CoL : Pfeffer 1894: 8; Boulenger 1898: 5, 23; Boulenger 1899: 54; Boulenger 1901a: 492, 499; Boulenger 1901b: 141; Moore 1903: 216; Boulenger 1905: 60; Boulenger 1906: 542, 576; Boulenger 1912: 199, 203; Boulenger 1916: 115, 141, Figure 96; Cunnington 1920: 529; David 1936: 158; Worthington and
Figure 4. Scatterplots of SL against (A) anterior border of snout to last, externally visible, dorsal spine (% SL); (B) anterior border of snout to last, externally visible; dorsal spine (% SL); (C) postanal length (% SL); and (D) body and depth (Z () % specimens SL). (•) Lectotype of Mastacembelus View in CoL , (O) paralectotypes polli View in CoL sp. nov. and Full (o line) specimens: fitted function of M. ophidium View in CoL for M. ophidium View in CoL ; (.) holotype., (Z) paratypes
Ricardo 1936 (in part): 1068, 1077, 1109; David and Poll 1937: 275; Poll 1946: 157, 245, 250–251; Hulot 1950: 172; Poll 1953: 9, 19, 236–237, 250, Plate 11 Figure 2 View Figure 2 ; Matthes 1962 (in part): 77–80; Bell-Cross and Kaoma 1971: 243; Brichard 1978: 75, 381 (two photographs), 438, 440; Bernacsek 1980: 62; Travers 1984a, 1984b (in part).
Afromastacembelus ophidium ( Günther, 1893) View in CoL : Travers 1984b: 145; Travers et al. 1986: 419; Eccles 1992: 84, 128, figure; Kawabata and Mihigo 1982: 138.
Caecomastacembelus ophidium ( Günther, 1893) View in CoL : Coulter 1991: 266; Abe 1997: 246–247, 249, 251, Figure 12-1a; Abe 1998: 273, 278; De Vos and Snoeks 1998: 31, Figure 2 View Figure 2 .
Aethiomastacembelus ophidium ( Günther, 1893) : De Vos et al. 1996: 17.
Type material
Lectotype (designated in this paper): BMNH 1889.1.30:22 (from 22–24); near Ujiji ( Tanzania) ( Udjidji ¡4 ° 569S, 29 ° 409E), coll. E. C. Hore (287 mm TL) . Paralectotypes (designated in this paper): BMNH 1889.1.30:23 (from 22–24) ; same data as for lectotype (three specimens, 191–208 mm TL) .
Since none of the type specimens has ever been illustrated (see recommendation ICZN 1999) the largest of the syntypes is here designated as the lectotype. Of the remaining four syntypes, three paralectotypes are here considered conspecific with the lectotype while the smallest paralectotype belongs to the new species described below. Worthington and Ricardo (1936) stated that the description of M. ophidium was based only on the larger syntypes. This can certainly be confirmed, for example, by the fact Günther (1893) gave a variation of 31 up to 32 dorsal spines for M. ophidium while the smallest syntype possesses only 23+1 dorsal spines. For more details see M. polli sp. nov.
Etymology
From the Greek ‘‘oQI δ Ion’’ (‘‘opidion’’) diminutive of the Greek ‘‘oQI ζ ’’ (serpent, reptile) referring to the snake-like appearance of this species.
Diagnosis
Within Lake Tanganyika, M. ophidium can be distinguished from all other species, except M. polli sp. nov., by a relatively long postanal length [54.1–60.5 (57.1)% SL versus 53.5%
Figure 5. Scatterplots of SL against (A) postanal length (% SL) and (B) distance from anterior border of snout to last, externally visible, anal spine (S-LAS) (% SL). (o) Mastacembelus albomaculatus ; (Z) M. cunningtoni ; (e)
M
M.. platysoma ellipsifer ;; (* (•)) M M.. flavidus polli sp;. nov (•) M.; (Z. micropectus ) M. tanganicae ; (.); and M. moorii (Ɨ) M;. (n zebratus ) M. ophidium .; (+) M. plagiostomus ; (O)
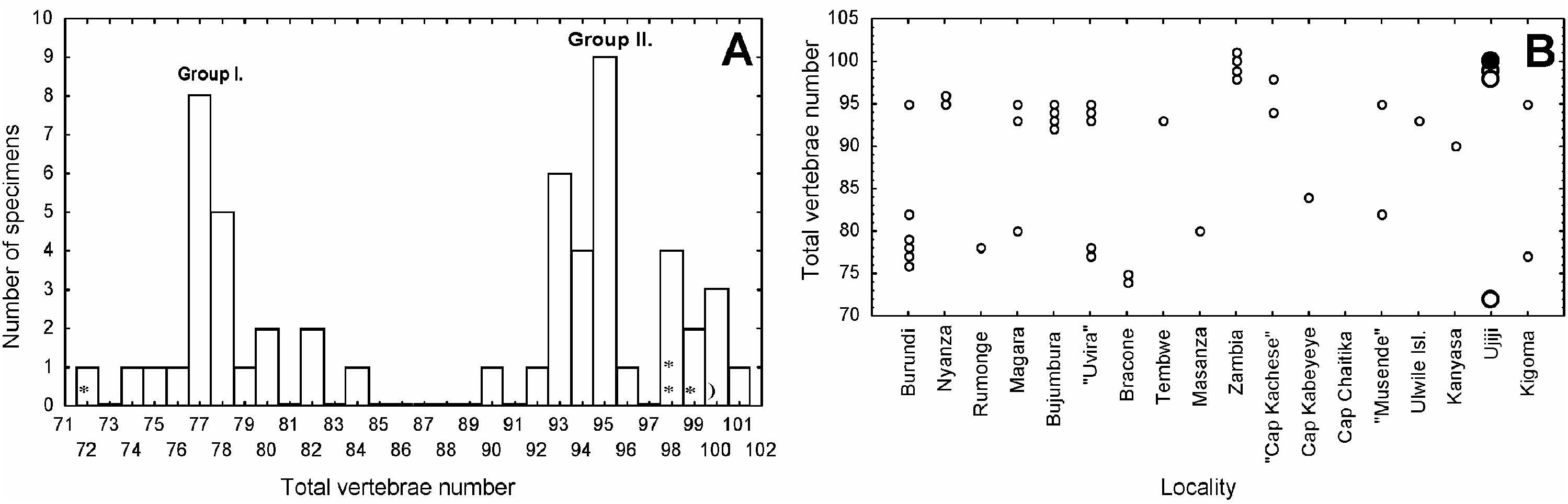
SL or less] increasing with size (Figure 5a), which is longer than the preanal length, itself being relatively short [38.3–45.0 (41.6)% SL versus 46.1% SL or more] and decreasing with size; by a relatively short distance from snout to last, externally visible, anal spine [40.0–46.6 (43.8)% SL versus 50.6% SL or more] (Figure 5b); and by its protruding eyes, protruding lower jaw, pointed caudal fin, posterior angle of lips situated below eye, from about one-third of the eye diameter, or even behind the posterior border of the eye (versus posterior angle of lips situated more anterior). From the highly similar M. polli sp. nov. it can be distinguished mainly by its greater dorsal spine number [27+1 to 33+1 (median 28+1) versus 21+1 to 28+1 (24+1)], its greater caudal vertebrae number [63–70 (66) versus 48–58 (53)]; and its related greater total vertebrae number [90–101 (95) versus 72–84 (77)].
Description
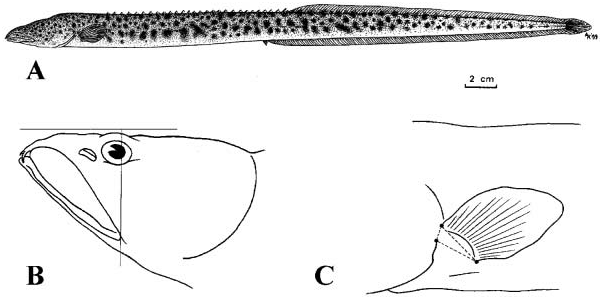
Meristics and morphometrics are given respectively in Tables III and IV. A representative specimen of this species is illustrated in Figure 6a–c View Figure 6 .
Mastacembelus ophidium has protruding eyes, a small rostral appendage, a protruding lower jaw, a pointed caudal fin and a relatively elongated pectoral-fin shape (i.e. not so rounded as in many other species). Posterior angle of lips situated below the region from the middle of the eye up to a distance of about one-third of eye diameter behind posterior border of eye. For the majority of the specimens the posterior angle of lips is situated below the posterior edge of the eye. Mastacembelus ophidium together with M. polli sp. nov. are the only African spiny eels in which the posterior angle of lips is situated so far posteriorly ( Figure 6b View Figure 6 ). Upper corner of gill opening and the dorsal edge of pectoral-fin base approximately at same level, clearly anterior to ventral edge of pectoral-fin base. Dorsal edge of pectoral-fin base situated above upper corner of the gill opening. Upper corner of gill opening situated between one-quarter and half (exceptionally three-quarters) of the vertical distance between the dorsal and ventral edge of the pectoral-fin base ( Figure 6c View Figure 6 ). Lateral line continuous from posterior border of head up to region of anus; further posteriorly, it becomes more and more discontinuous.
Preanal length always shorter than postanal length; distance from anterior border of snout to last externally visible dorsal spine always longer than distance from anterior border of snout to last externally visible anal spine, and consequently origin of soft dorsal fin always posterior compared to origin of soft anal fin.
A high number of dorsal spines, XXVII+I to XXXIII+I, with spines increasing in size from first to last. Usually a very small, almost entirely reduced spine hidden under the skin, and situated anterior to the base of the first dorsal-fin ray. Nevertheless, the dorsal spine formula is standardized as X+I.
One well-developed, externally visible, anal spine. In addition, a very small almost entirely reduced spine, hidden under the skin, and situated anterior to the base of the first anal-fin ray can be present. First anal pterygiophore well developed, supporting only the first anal spine. Second anal pterygiophore very small, sometimes supporting an almost entirely reduced anal ‘‘spine’’. Nevertheless, the anal spine formula is standardized as I+I.
In all specimens the neural spine-supporting pterygiophore of the last externally visible dorsal spine and the haemal spine-supporting pterygiophore of the first anal spine are situated on two different vertebrae and are separated by one to three vertebrae (named in-between vertebrae hereafter). The vertebra with the neural spine supporting the pterygiophore of the last externally visible dorsal spine is always situated posterior to the vertebra whose haemal spine supports the first anal spine.
All specimens lack preopercular and preorbital spines.
Maximal observed standard length: 406 mm (422 mm TL).
Coloration (see also Figure 6a View Figure 6 )
Based on MRAC 75-01-P-119–123 unless otherwise stated. Uniformly light brown background colour with generally numerous small, round, dark brown spots on lateral sides and back of head, body and tail. Spots may be far less abundant or even absent on entire tail, or more posterior part of tail. Exceptionally, spots restricted to head region ( MRAC 92-081-P-1441). Further, spots mainly limited to three series, one on the dorsal midline and one on each lateral line forming nearly continuous bands, especially on the tail region ( MRAC 90973). In another specimen spots found on each side of dorsal midline and on anterior part of dorsal fin base. Remaining spots far less contrasted with the background colour than in other specimens examined (see also MRAC 85-12-P-7). Background colour lighter, more yellowish white on lips, ventral region of head, belly and most ventral part of tail. Pectoral fins whitish transparent without spots or eventually only spotted at their base. Dorsal fin light brown with a series of numerous small, round, dark brown spots at its base, outer margin white. Caudal fin light brown at its base and yellowish white towards its outer margin. Anal fin yellowish white.
Distribution (see Figure 7)
Mastacembelus ophidium is endemic to Lake Tanganyika and confirmed locality records indicate a circumlacustrine coastal distribution. However, at present, it has not been found over large parts of the Democratic Republic of Congo coastline, but this part of the lake is
Figure 7. Geographical distribution of Mastacembelus ophidium based on the localities of the examined specimens. (•) Lectotype and paralectotypes, and (•) specimens of M. ophidium .
poorly sampled. Kawabata and Mihigo (1982) reported M. ophidium from around the Ruzizi River estuaries.
The species is reported to be rare ( Poll 1953).
Generic status
Günther (1893) described M. ophidium as a new member of the genus Mastacembelus . Travers (1984b) placed M. ophidium within the genus Afromastacembelus (see also Travers et al. 1986). Travers (1988) revealed that the type species of the genus Afromastacembelus , A. tanganicae ( Günther, 1893) in fact belongs to the genus Caecomastacembelus and created a new genus Aethiomastacembelus to allocate most of the species previously in Afromastacembelus . However, Travers (1988) did not mention to which genus M. ophidium was allocated. Subsequently, Coulter (1991) and Abe (1997, 1998) placed it in the genus Caecomastacembelus . Vreven and Teugels (1996) revealed several inaccuracies and contradictions between the type material and the diagnosis of both genera. Vreven (forthcoming) placed Caecomastacembelus and Aethiomastacembelus in synonymy with Mastacembelus .
Based on the meristic, morphometric and colour pattern evidence M. ophidium seems to be most closely related to M. polli sp. nov. The more distant affinities of both species remain, at present, unresolved and need additional research.
Biology and ecology
Note. The literature data on M. ophidium provided here need to be handled with care as M. ophidium and M. polli sp. nov. have not been distinguished in the past. Therefore, misidentification of specimens mentioned in the literature can certainly be expected (see Synonyms and Citations).
Habitat. Poll (1953) mentioned M. ophidium occurring in coastal regions of the lake up to a depth of 10 m. Matthes (1962) reported two specimens (verified) as M. ophidium from rocky bottoms. However, most of the other specimens identified by himself as M. ophidium from rocky bottoms are M. polli sp. nov. (see below). Also Brichard (1978) reported the species living in rocky habitats. However, Travers et al. (1986) and Eccles (1992) reported that the species inhabits sandy shores. In addition, M. ophidium was reported as a sanddwelling species occasionally found on rocky slopes (sand/rock) by Brichard (1989). Finally, Abe (1997) also reported that M. ophidium occupies sandy bottoms. Hence, M. ophidium is most probably a sand-dwelling species occasionally found on rocky bottoms (see also under Discussion).
It is well known that sand-dwelling fluviatile species of spiny eels bury themselves in the sand to lay in ambush waiting for prey to pass by, or to do so as a protection against predators ( Brichard 1989). Brichard (1989) suggested that it would not be surprising to find also that sand-dwelling Lake Tanganyika species bury themselves in the sand ( Brichard 1989). Indeed, this burying and ambush behaviour was confirmed and illustrated by Jäger (2002) based on aquarium observations.
De Vos et al. (1996) reported M. ophidium from the sub-littoral (10–40 m depth) as well as from the deeper benthic (40–60 m depth) environment.
Food. Worthington and Ricardo (1936) mentioned that one specimen had been feeding on small prawns. Poll (1953) reported the presence of one Lamprologus sp. of 5 cm in the stomach of one of the specimens studied by himself. Indeed, based on X-ray data of many specimens, the presence of fish(es) in the stomach of some of the examined specimens is confirmed.
Reproduction. Vast numbers of M. ophidium fry have been noted periodically near the shore at the north of the lake ( Coulter 1991), indicating mass spawning ( Brichard 1978). It is the only species from which concentrations of thousands of young fry a few centimetres long have been observed in quiet bays during some months of the year ( Brichard 1989). Following Brichard (1989) it therefore appears that the spiny eels might migrate and have synchronous spawning, but as yet this observation applies only to M. ophidium and not to any other species (see also below under Discussion).
Poll (1953) reported an immature male ( MRAC 90973, 349 mm TL, 20 December 1946) and a mature female ( ISNB 9431, 332 mm SL, 25 January 1947). Other specimens ( MRAC 91643, 400 mm TL, 3 November 1949; MRAC 92-081 View Materials -P- 1441, 341 mm TL, 1 June 1992) are here identified as a ‘‘nearly ripe’’ females. Based on these reported data it is obvious that additional specimens will be necessary to identify reproduction period(s) .
Abe (1998) reported that the oocytes of M. ophidium are small when compared to the oocytes of M. albomaculatus , M. micropectus , M. plagiostomus , and M. tanganicae which have an oocyte diameter larger than or equal to 1.5 mm. Nevertheless, for both specimens I examined ( MRAC 91643, 400 mm TL; MRAC 92-081-P- 1441, 341 mm TL) the egg diameter is around 1.5 mm.
Fisheries
Mastacembelus ophidium View in CoL is of little value as food ( Poll 1953; Eccles 1992). According to Eccles (1992), their shape makes them difficult to net, although they can be taken with a small hook. Eccles (1992) mentioned that M. ophidium View in CoL might be of some interest to aquarists. Indeed, M. ophidium View in CoL is presently available in Germany (www.pet2get.dk/ stockliste 2003).
Other specimens examined
All specimens originated from Lake Tanganyika. For samples with more than one specimen and without separate numbering the exact number is provided. All lengths are total lengths.
Country unknown. BMNH 2003.3.23:3 (from 1919.7.24:35–42), ditch near Lake Tanganyika (¡ 323 mm) . BMNH 1936.6.15:1753 (from 1753–1756) (170 mm) . BMNH 1936.6.15:1757, Lake Tanganyika (¡ 296 mm) .
Burundi. MCZ 50841, between Mutumba and Magara among rocks, depth 0–10 m (¡3 ° 409S, 29 ° 209E) (two specimens, 213–254 mm). MRAC 39044–045 View Materials , Nyanza (¡4 ° 209S, 29 ° 389E) (156–185 mm). MRAC 73-68 View Materials -P-550, Bujumbura (¡3 ° 239S, 29 ° 229E) (229 mm). MRAC 75-01 View Materials -P-119–123, 8 km au Sud de Bujumbura (¡3 ° 239S, 29 ° 229E) (229–326 mm). MRAC 76-09 View Materials -P-216, côte du Burundi (204 mm). MRAC 85-12 View Materials -P-7, Bujumbura (¡3 ° 239S, 29 ° 229E) (422 mm) .
Democratic Republic of Congo. BMNH 1906.7.8:278, Burton Gulf (¡4 ° 129S, 29 ° 089E) (315 mm) . BMNH 1968.12.30:4, Kirambo Lagoon (¡7 ° 259S, 30 ° 369E) (¡ 130 mm) (cleared and stained) . MRAC 90973 View Materials , Stat. 27, Baie de Tembwe, le long de la rive Sud, sur la plage, senne (¡6 ° 319S, 29 ° 289E) (349 mm) . MRAC 91643 View Materials , Uvira (¡3 ° 249S, 29 ° 089E) (400 mm) . MRAC 93639 View Materials , Uvira (¡3 ° 249S, 29 ° 089E) (271 mm) . MRAC 130379–380 View Materials , Uvira , digue I . R.S.A.C. (¡3 ° 249S, 29 ° 089E) (I. R.S.A.C.) (326–395 mm).
Tanzania. BMNH 1982.4.13:4821, Karago Bay (¡5 ° 169S, 29 ° 489E) (176 mm) . IRSNB 9431 View Materials , Baie de Karago, senne, Stat. 89 (¡5 ° 169S, 29 ° 489E) (345 mm) . MRAC 92-81 View Materials -P- 1441, Kanyasa (¡5 ° 569S, 29 ° 549E) (341 mm) . MRAC 92-81 View Materials -P-1442, Ulwile Island , northern shore (¡7 ° 27940S, 30 ° 34920E) (274 mm) . SAIAB 56007 About SAIAB , Kigoma, Kigoma Bay below hill to Hotel (4 ° 539030S, 29 ° 379110E) (230 mm) .
Zambia. MRAC 78-25 View Materials -P-39, Cap Chaitika (¡8 ° 349S, 30 ° 489E) (183 mm) . MRAC 78-25 View Materials -P-40, Cap Kachese (¡8 ° 299S, 30 ° 299E) (220 mm) . ROM 28166 (two specimens, 229–270 mm) ; ROM 28181, Lake Tanganyika (¡?) (three specimens, 204–296 mm) . SAIAB 41260 About SAIAB , Kombe (¡8 ° 499S, 31 ° 089E) (153 mm) . SAIAB 42334 About SAIAB , Ndole Bay (¡8 ° 299S, 30 ° 289E) (323 mm) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Mastacembelus ophidium Günther, 1893
| Vreven, E. J. 2005 |
Aethiomastacembelus ophidium ( Günther, 1893 )
| De Vos L & Nshombo M & Thys van den Audenaerde DFE 1996: 17 |
Caecomastacembelus ophidium ( Günther, 1893 )
| Abe N 1998: 273 |
| De Vos L & Snoeks J 1998: 31 |
| Abe N 1997: 246 |
| Coulter GW 1991: 266 |
Afromastacembelus ophidium ( Günther, 1893 )
| Eccles DH 1992: 84 |
| Travers RA & Eynikel G & Thys van den Audenaerde DFE 1986: 419 |
| Travers RA 1984: 145 |
| Kawabata M & Mihigo NYK 1982: 138 |
Mastacembelus ophidium Günther, 1893
| David L 1936: 158 |
| Cunnington WA 1920: 529 |
| Boulenger GA 1916: 115 |
| Boulenger GA 1912: 199 |
| Boulenger GA 1906: 542 |
| Boulenger GA 1905: 60 |
| Moore JES 1903: 216 |
| Boulenger GA 1901: 492 |
| Boulenger GA 1901: 141 |
| Boulenger GA 1899: 54 |
| Boulenger GA 1898: 5 |
| Pfeffer GJ 1894: 8 |