Myriangiales, K.Starback, 1899
|
publication ID |
https://doi.org/10.11646/phytotaxa.176.1.13 |
|
persistent identifier |
https://treatment.plazi.org/id/03B487E8-9E4E-CA59-FF3D-FF4C543BFD32 |
|
treatment provided by |
Felipe |
|
scientific name |
Myriangiales |
| status |
|
Myriangiales View in CoL View at ENA
The order Myriangiales was introduced by Starbäck (1899) for the species characterized by having crustose ascostromata and muriform ascospores, based on the type species, Myrangium duriaei Mont. & Berk. ( Miller 1938, Hyde et al. 2013). Multigene phylogenetic studies revealed that the order Myriangiales always clusters in Dothideomycetes ( Boehm et al. 2009, Schoch et al. 2009, Zhang et al. 2012, Hyde et al. 2013). This order is characterized by pulvinate, irregular ascostromata in which the asci are irregularly arranged in one or more layers in locules. Locules may contain single or multiple asci within each locule. Asci have a minute pedicel and indistinct ocular chambers. Ascospores are irregularly arranged and are liberated only by the breakup of the stromatal layers above them. Asexual states are coelomycetous. Kirk et al. (2008) included three families under order Myriangiales , Cookellaceae , Elsinoaceae and Myriangeaceae . Lumbsch & Huhndorf (2010) accepted only Elsinoaceae and Myriangeaceae in Myriangiales based on phylogenetic results. Elsinoaceae is morphologically different from Myriangeaceae as 3 to 10 asci form in locules in light coloured pseudoascostromata, which form typical scab-like blemishes on leaf or fruit surfaces. No molecular data is available for Cookellaceae and its placement in Myriangiales cannot be confirmed.
Elsinoaceae Höhn. ex Sacc. & Trotter [as 'Elsinoёaceae'], Sylloge Fungorum (Abellini) 22: 584 (1913)
Synonyms:
Myxomyrangiaceae (Theiss.) Theiss.
Plectodiscellaceae Woron., Mykol. Zentbl. 4: 232 (1914)
Saccardinulaceae G. Arnaud, Annls Sci. Nat., Bot. , sér. 10 7: 647 (1925)
Parasitic or saprotrophic on plant leaves and fruits causing scab and sunken scab-like blemishes. Sexual state: Pseudoascostromata usually spread around host veins, solitary, aggregated, or gregarious, wart-like or scab-like blemishes, pulvinate, superficial, globose to subglobose, white, pale yellow to brown, multi-loculate, locules scattered in upper part of pseudoascostromata. Cells of pseudoascostromata comprising host cells and interdispersed light coloured fungal hyphae, opening by unordered break down of the surface layer. Locules with 3–10 asci inside each locule, ostiolate. Ostioles minute. Pseudoparaphyses absent. Asci 8-spored, bitunicate, fissitunicate, saccate to globose, with a minute pedicel, and indistinct ocular chamber. Ascospores irregularly arranged, oblong or fusiform with slightly acute ends, with 2–3 transverse septa, hyaline, smooth-walled, lacking a sheath. Asexual state: coelomycetous “ Sphaceloma ”. Lesions circular, dark brown raised margin, cream-brown. Acervuli subepidermal, pseudoparenchymatous. Conidiophores hyaline to pale-brown, polyphialidic. Conidiogenous cells formed directly from the upper cells of the pseudoparenchyma, monophialidic to polyphidalic, integrated or discrete, determinate, hyaline to pale brown, lacking a thickened region around the phialide channel. Conidia hyaline, unicellular, ellipsoidal, aseptate, biguttulate.
Lumbsch & Huhndorf (2010) included ten genera in Elsinoaceae . Li et al. (2011), Hyde et al. (2013) and Dissanayake et al. (2014) however, revised the familial positions of Beelia , Butleria , Hyalotheles , Hemimyriangium , Saccardinula , Stephanotheca and Xenodium and supported the separation of Elsinoaceae from the family Myriangiaceae based on phylogenetic analysis. The families are also morphologically distinct. In Elsinoaceae several asci form in locules that develop in pseudoascostromata, that comprises host cells and interdispersed light coloured fungal hyphae. In Myriangiaceae , asci develop singly in locules that are generally scatted in ascostromata. The pseudoascostromata of Elsinoaceae are generally superficial, pulvinate and comprise of light coloured fungal hyphae and darkened host cells. Saccardinula shows similar characters to the family Brefeldiellaceae where it is placed ( Hyde et al. 2013). Stephanotheca shows characteristic of the family Asterinaceae where it is retained, whereas Xenodium has unitunicate asci and thus excluded from Dothideomycetes. Hyalotheles has been placed in Dothideomycetes genera incertae sedis. Butleria and Hemimyrangium are placed in the family Myriangeaceae by Dissanayake et al. (2014). Fresh collections are needed for epitypification and obtaining sequence data.
Type:— Elsinoe Racib., Parasitische. Algen und Pilze View in CoL Java’s ( Jakarta) 1: 14 (1900)
Possible synonyms
Sphaceloma de Bary, Ann. Oenol. 4: 165 (1874)
Bitancourtia Thirum & Jenkins, Mycologia View in CoL 45(5): 781 (1953)
Isotexis Syd. , in Sydow & Petrak, Annls mycol. 29 (3/4): 261 (1931)
Plectodiscella Woron., Mykol Zentbl 4: 232 (1914)
Uleomycina Petr., Sydowia View in CoL 8(1–6): 74 (1954)
Kurosawaia Hara, List of Japanese Fungi: 172. Ed. 4 (1954)
Manginia Viala & Pacotter , C.r. hebd, Séanc. Acad. Sci., Paris 139: 88 (1904)
Melanobasidium Maubl., Bull. Soc. Mycol. Fr. View in CoL 22: 69 (1906)
Melonobasis Clem. & Shear, Gen. fung., Edn 2 (Minneapolis): 224, 403 (1931)
Melanodochium Syd., Annls mycol. 36 (4): 310 (1938)
Melanophora Arx, Verh. K. ned, Akad, wet., tweede sect. 51(3): 43 (1957)
P aras it ic on pl ant leav e s and frui t s caus ing scab and sunk en scab- li k e bl em i shes. S ex ual st at e: Pseudoascostromata usually spread around host veins, solitary, aggregated, or gregarious, wart-like or scab-like blemishes, pulvinate, superficial, globose to subglobose, white, pale yellow to brown, multi-loculate, locules scattered in upper part of pseudoascostromata. Cells of pseudoascostromata comprising host cells and interdispersed light coloured fungal hyphae opening by unordered break down of the surface layer. Locules with numerous 3–8 asci inside each locule, ostiolate. Ostiole minute. Pseudoparaphyses absent. Asci 8-spored, bitunicate, fissitunicate, saccate to globose, apedicellate, with indistinct ocular chamber. Ascospores irregularly arranged, oblong or fusiform with slightly acute ends, with 2–3 transverse septa, hyaline, smooth-walled, lacking a sheath. Asexual state: Ceolomycetous “ Sphaceloma ” Acervuli subepidermal, pseudoparenchymatous. Conidiophores hyaline to pale-brown, polyphialidic. Conidiogenous cells formed directly from the upper cells of the pseudoparenchyma, monophialidic to polyphialidic, lacking a thickened region around the phialidic channel, terminal, integrated, determinate, hyaline to pale brown. Conidia hyaline, unicellular, ellipsoidal, aseptate, biguttulate.
Type species:— Elsinoe canavaliae Racib. View in CoL [as ' canavalliae '], Parasitische. Algen und Pilze Java’s ( Jakarta) 1: 14
(1900)
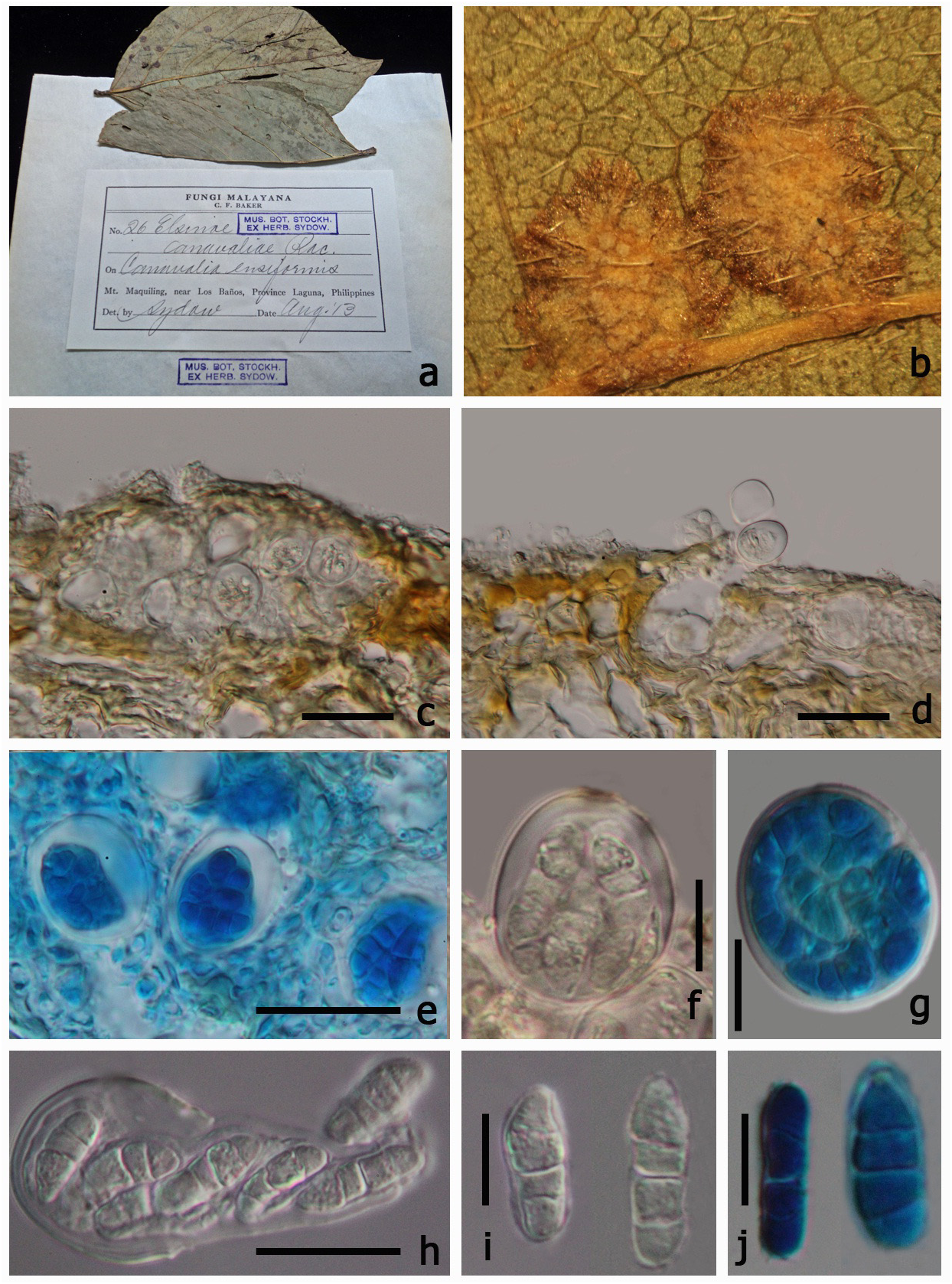
≡ Uleomyces canavaliae (Racib.) G. Arnaud, Annales des Sciences Naturelles Botanique 10 5: 685 (1925) MycoBank: 217658 ( Figs 2 View FIGURE 2 , 3 View FIGURE 3 )
Parasitic on leaves, forming scab on lower leaf surface. Sexual state: Pseudoascostromata 1–5 × 5–8 mm in diam. ( x = 3.2 × 6.5 mm, n=10), spreading around the host veins, solitary, aggregated, or gregarious, wart-like or scablike blemishes, pulvinate, superficial, globose to subglobose, white, pale yellow or occassionally brown, in section with numerous locules distributed inside the upper part of pseudoascostromata, with numerous asci within each locule. Cells of pseudoascostromata comprising host cells and inter-dispersed light coloured fungal hyphae. Locules with 3–8 asci inside each locule, ostiolate. Ostiole minute. Pseudoparaphyses absent. Asci 16–22 ×16–21 µm ( x = 19.7 × 18.9 µm, n=20), 8-spored, bitunicate, fissitunicate, saccate to globose, apedicellate, with indistinct ocular chamber. Ascospores 10–14 × 3–5 µm ( x = 12.3 × 4 µm, n=40) irregularly arranged, oblong or fusiform with slightly acute ends, with 2–3 transverse septa, hyaline, smooth-walled, lacking a sheath. Asexual state: “ Sphaceloma View in CoL ”, Acervuli sub-epidermal, pseudoparenchymatous. Conidiophores hyaline to pale-brown, polyphialidic. Conidiogenous cells formed directly from the upper cells of the pseudoparenchyma, monophialidic to polyphidalic, integrated or discrete, determinate, hyaline to pale brown, lacking a thickened region around the phialide channel. Conidia 8–16 × 3–5 µm ( x = 14.1 × 4 µm, n = 20), hyaline, unicellular, ellipsoidal, aseptate, biguttulate.
Material examined:— Philippines. Laguna Province: Mount Maquiling, near Los Baños, on Canavalia ensiformis (Fabaceae) , Baker, August 1913 (S, F66900 View Materials !, isotype).
Elsinoe was established by Raciborski (1900) including three species ( E. canavaliae , E. antidesmae Racib. , E. meninspermacearum Racib. ). von Arx & Müller (1975) placed Elsinoe in Myriangiaceae based on its immersed or erumpent, pulvinate or irregular ascomata and being parasitic on higher plants causing scab. Later, the genus was placed in the family Elsinoaceae ( Barr 1979, Kirk et al. 2001, Lumbsch & Huhndorf 2007, 2010). There are 139
126 Phytotaxa 176 (1) © 2014 Magnolia Press JAYAWARDENA ET AL.
species epithets for Elsinoe (Index Fungorum 2013) and they are generally parasites on leaves, stems, and fruits. The asexual state of Elsinoe is “ Sphaceloma ” (Wijayawardena et al. 2012). Bitancourt & Jenkins (1946) described Sphaceloma manihoticola Bitanc. & Jenkins on Manihot esculenta Crantz ( Zeigler & Lozano, 1983) . The relationship between Sphaceloma and Elsinoe has been resolved by analyzing rDNA sequence data ( Cheewangkoon et al. 2010). There are 168 species epithets for Sphaceloma in Index Fungorum (2013). Most of the Sphaceloma species are pathogens that affect flowers, fruits, leaves and stems, causing characteristic scab lesions on the organs that they attack as well as necrotic spots on leaves.
Elsinoe is an important plant pathogenic genus causing scab and anthracnose. This genus is widely known in association with various Citrus species. But it also causes diseases in Malus , Rubus , Vitis species and several other plants and effects plant families such as Moraceae , Piperaceae , Sapindaceae , Anacardiaceae , Myrtaceae and Vitaceae . Elsinoe fawcettii Bitanc. & Jenkins and E. australis Bitanc. & Jenkins cause scab disease of Citrus sp. ( Hanlin 1989, Timmer et al. 1996, Hyun et al. 2001, Nelson 2008) and there are many other important pathogens of Elsinoe and its asexual states ( Table 3). Elsinoe takoropuku G.S. Ridl & Ramsfield is a recently introduced species ( Ridley & Ramsfield 2006), but differs from E. canavaliae markedly as it forms ascostromata on twigs of Pittosporum tenuifolium Gaertn instead of scabs on leaves. It contains locules each containing single asci. The asci are however, thought to be more similar to Elsinoe type even though it has many characters similar with Myriangiaceae .
128 Phytotaxa 176 (1) © 2014 Magnolia Press JAYAWARDENA ET AL.
Species named as Sphaceloma should be synonymized under Elsinoe if their relationships are confirmed by molecular data
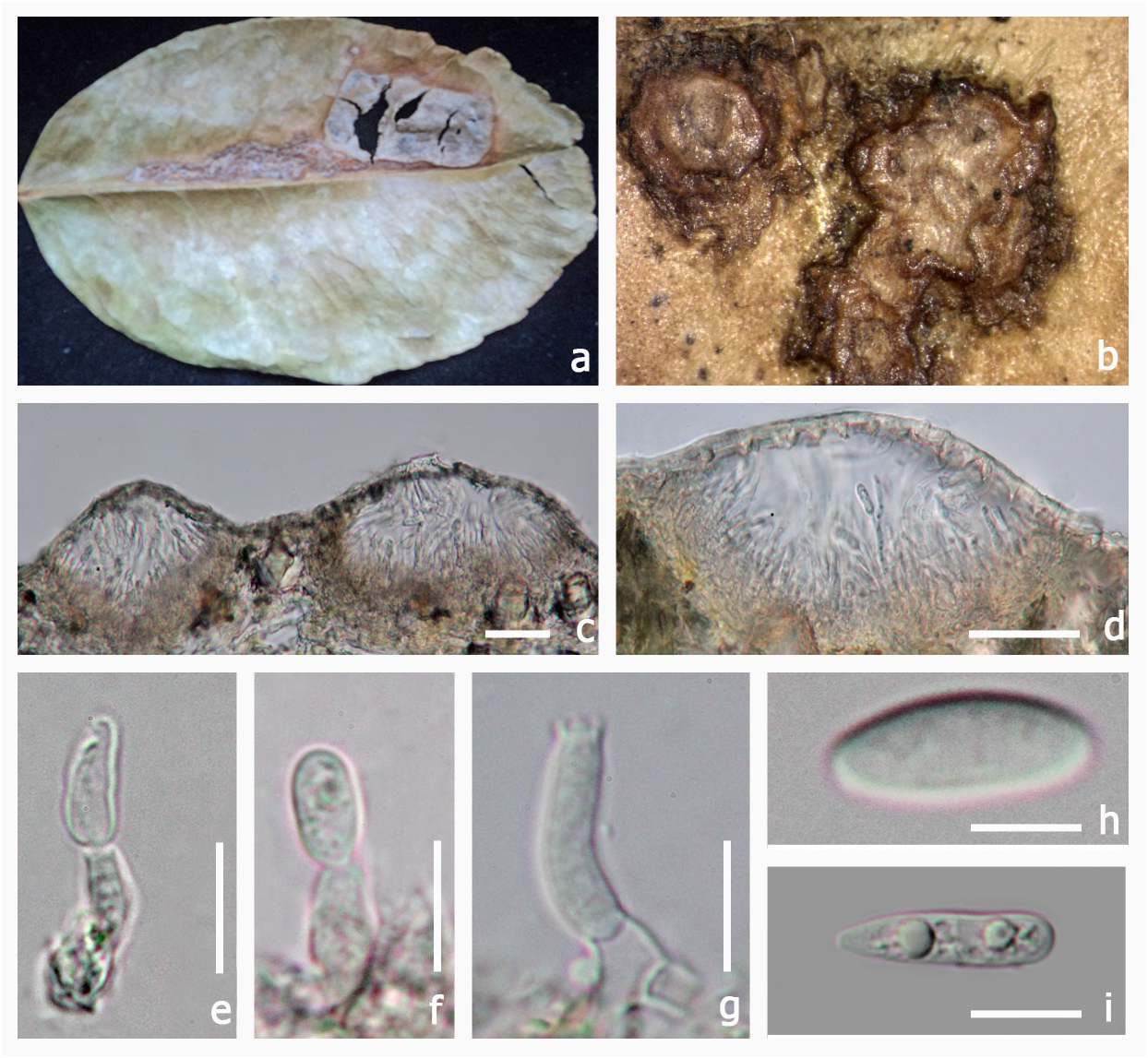
Schoch et al. (2006, 2009) and Boehm et al. (2009) showed that there is an obvious subclade among the species of Myriangiaceae (named Elsinoaceae ). However, only four Elsinoe strains and one Myriangium strain were used in Schoch et al. (2006) and no Elsinoe strains were used in Schoch et al. (2009). Thus, molecular data does convincingly resolve the two families. In the study on the taxonomy of the species associated with scab disease of Proteaceae, Swart et al. (2001) analyzed ITS sequence of six Elsinoe species , E. banksiae Pascoe & Crous , E. leucospermi L. Swart & Crous , E. proteae Crous & L. Swart , Elsinoe sp. (from Citrus ), Elsinoe sp. (from Banksia ), Sphaceloma protearum L. Swart & Crous ; the molecular analysis supported five species and four were described in their paper. Hyun et al. (2001) have done molecular analysis of several Elsinoe isolates causing scab disease of Citrus sp. in Jeju Island in Korea. Kerry et al. (2011) used molecular identification for Sphaceloma perseae and its absence. Phylogenetic analysis in for this paper shows that E. veneta and E. brasiliensis clustered separately from the Elsinoaceae clade. The basionym of Elsinoe veneta is Plectodiscella veneta Burkh. Jenkins (1932) however, considered Plectodiscella veneta to be a species of Elsinoe and transferred it to E. veneta . In Elsinoe veneta and E. brasiliensis the pseudoascostromata are multi-loculate, pulvinate, scab-like structures, asci are globose, bitunicate and fissitunicate and ascospores hyaline with three septa which resembles Elsinoaceae . In Elsinoe veneta and E. brasiliensis there is one ascus per locule (Saccardo 1925–1928). In the type genus Elsinoe , multiple asci can be found in each locule ( Hyde et al. 2013). Further studies are needed to clarify their taxonomic placement.
Several studies have been conducted on Elsinochrome which are non-host selective, light-activated, polyketide-derived toxins produced by Elsinoe species ( Chung & Liao 2008). Further molecular studies are needed on Elsinoe species and also on its asexual state to resolve the correct placement of this taxon. Phylogenetic analysis shows that Sphaceloma species cluster with Elsinoe species ( Fig. 1 View FIGURE 1 ) and this is also supported by morphological data ( Cheewangkoon et al. 2010). Based on the available molecular and morphological data it may be necessary to synonymize Sphaceloma under Elsinoe . However, the type species of Elsinoe nor Sphaceloma have been sequenced and therefore they should not be synonymized at this time. Therefore we list Sphaceloma as a possible synonym only.
Molleriella G. Winter, Boletim da Sociedade Broteriana, Coimbra View in CoL , sér 1, 4: 199 (1886)
Possible synonyms:
Agrona H ӧhn., Sber, Akad, Wien, Math-naturw, Kl., Abt. 1, 118: 362 [88 repr.] (1909)
Capnodiopsis Henn., Hedwigia 41: 298 (1902)
Elachophyma Petr. , in Sydow & Petrak, Annls. Mycol. 29 (3/4): 258 (1931)
Elenkinella Woron., Bot. Mater. Insr. Sporov. Rast, Glavn, Bot. Sada RSFSR 1: 33 (1922)
Nostocotheca Starbäck, Bih, K. svenska VetenskAkad. Handl., Afd. 3 25 (no. 1): 20 (1899)
Saprotrophic on plant leaves causing scab-like lesions on lower leaf surface. Sexual state: Pseudoascostromata solitary, aggregated or gregarious, pulvinate, superficial, globose to oval, pale yellow, multi-loculate, locules scattered in upper part of pseudoascostromata. Cells of pseudoascostromata comprising host cells and interdispersed light coloured fungal hyphae opening by unordered break down of the surface layer. Locules with 4–10 asci inside each locule, ostiolate. Ostiole minute, Paraphyses absent. Asci 8-spored, bitunicate, fissitunicate, globose to subglobose, thick-walled, with a minute pedicel and an ocular chamber. Ascospores irregularly arranged, oblong to sub-clavate, 6–8-septate, hyaline. Asexual state: Unknown.
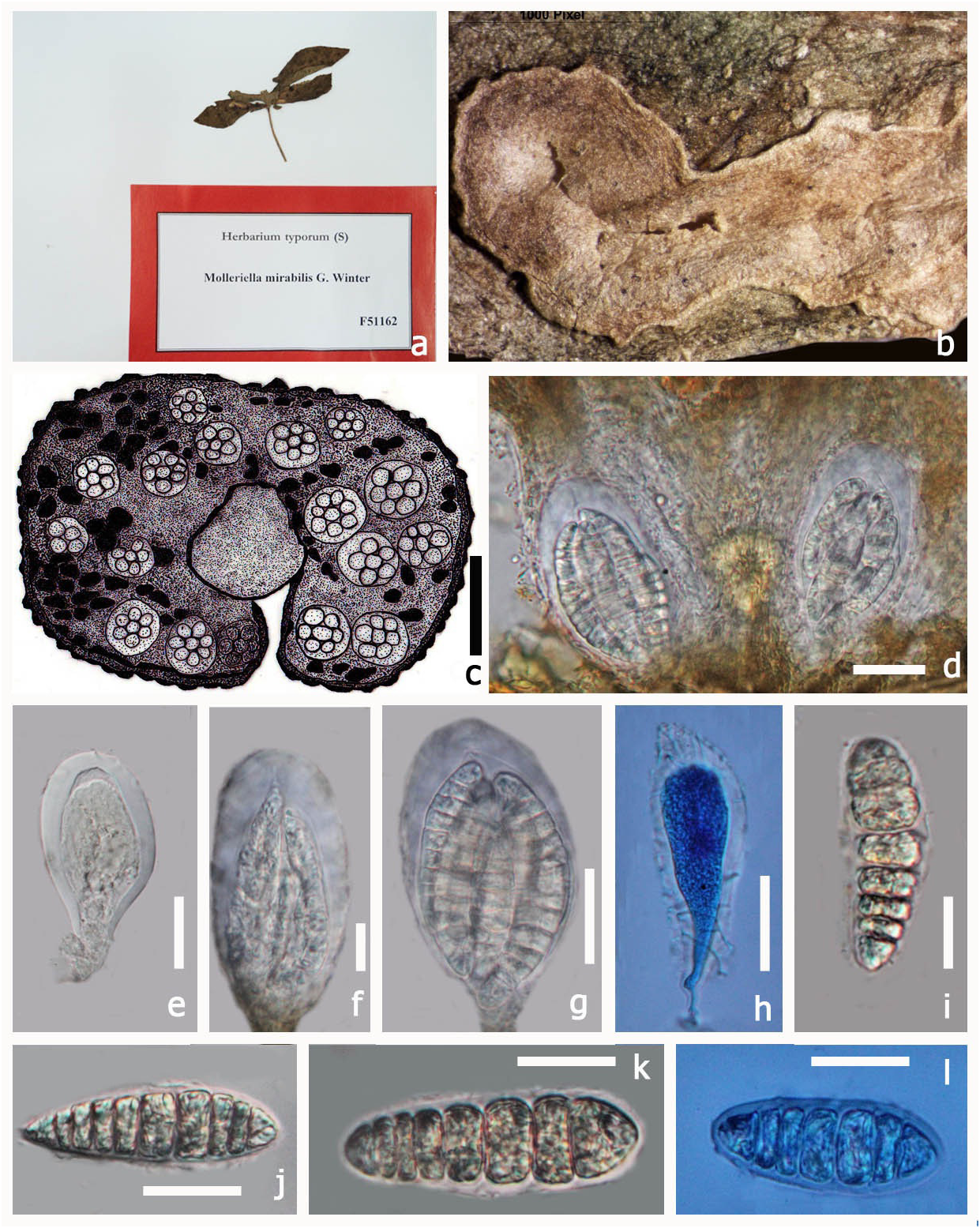
Type: — Molleriella mirabilis G. Winter, Boletim da Sociedade. broteriana, Coimbra, sér 1, 4: 199 (1886) MycoBank No: 528389 ( Fig 4 View FIGURE 4 )
Saprotrophic on plant leaves. Sexual state: Pseudoascostromata 5–8 × 2–3 mm ( x = 7.5 × 3.1 mm, n = 5) spread on lower surface, solitary, aggregated, or gregarious, pulvinate, superficial, pale yellow, multi-loculate, locules scattered in the upper part of the pseudoascostromata. Cells of pseudoascostromata comprising host cells and interdispersed light coloured fungal hyphae. Locules with 4–10 asci inside each locule, ostiolate. Ostiole minute.
130 Phytotaxa 176 (1) © 2014 Magnolia Press JAYAWARDENA ET AL.
Paraphyses not observed. Asci 17–37 × 9–21 µm ( x = 31.4 × 16.1 µm, n = 10) 8-spored, bitunicate, fissitunicate, globose to sub globose, solitary or aggregated, with minute pedicel and an ocular chamber. Ascospores 18–24 × 6–8 µm ( x = 22.7 × 7.3 µm, n = 10) irregularly arranged, smooth, oblong to sub-clavate, often curved, 6–8-septate, muriform, hyaline, no sheath. Asexual state: Unknown.
Material examined:— AFRICA. S. Thomé Insel, pr. Bate-pá, on Convolvulaceae, A. Moller , June 1885 (S, F51162 View Materials !, type)
The genus Molleriella was introduced by G. Winter (1886) in the class Discomycetes and later placed in the family Phymatophaeriaceae by Engler et al. (1887, 1897) and Hieronymus and Hennings (1901). Boedijn (1961) placed Molleriella in the family Saccardiaceae , while Arnaud (1918) and Bessy (1950) had placed Molleriella in Myriangiaceae . On the other hand, Kirk et al. (2001) placed Molleriella in the family Elsinoaceae and Lumbsch & Huhndorf (2007, 2010) followed this. At present there are four species epithets listed in Index Fungorum (2013). This genus differs from the type Elsinoe by being saprotrophic and differs in having globose to subglobose sessile asci. This genus is similar to family type Elsinoe in having multi-loculate, pulvinate scab-like blemish pseudoascostromata, without paraphyses, and minute ostioles, plus globose asci with indistinct ocular chambers. This genus is retained in the family Elsinoaceae but, fresh collections and molecular analysis are needed to establish natural placement of this genus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
Myriangiales
| Jayawardena, Ruvishika S., Ariyawansa, Hiran A., Singtripop, C., Li, Yan Mei, Yan, Jiye, Li, Xinghong, Nilthong, S. & Hyde, Kevin D. 2014 |
Bitancourtia
| Bitancourtia Thirum & Jenkins 1953: 781 |
Elenkinella Woron., Bot. Mater. Insr. Sporov. Rast, Glavn, Bot. Sada
| Rast, Glavn 1922: 33 |
Plectodiscella
| Plectodiscella Woron. 1914: 232 |
Sylloge Fungorum (Abellini)
| 1913: 584 |
Melanobasidium Maubl., Bull. Soc. Mycol. Fr.
| Melanobasidium Maubl. 1906: 69 |
C.r. hebd, Séanc. Acad. Sci., Paris
| 1904: 88 |
Capnodiopsis
| Capnodiopsis Henn. 1902: 298 |
Elsinoe
| Racib., Parasitische. Algen und Pilze 1900: 14 |
Molleriella
| G. Winter, Boletim da Sociedade Broteriana 1886: 199 |