Oregodasys katharinae, Hochberg, Rick, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.199011 |
|
DOI |
https://doi.org/10.5281/zenodo.6208808 |
|
persistent identifier |
https://treatment.plazi.org/id/03957E37-FFC7-C66E-FF5B-FED5842EBD6A |
|
treatment provided by |
Plazi |
|
scientific name |
Oregodasys katharinae |
| status |
sp. nov. |
Oregodasys katharinae View in CoL sp. nov.
( Figs. 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
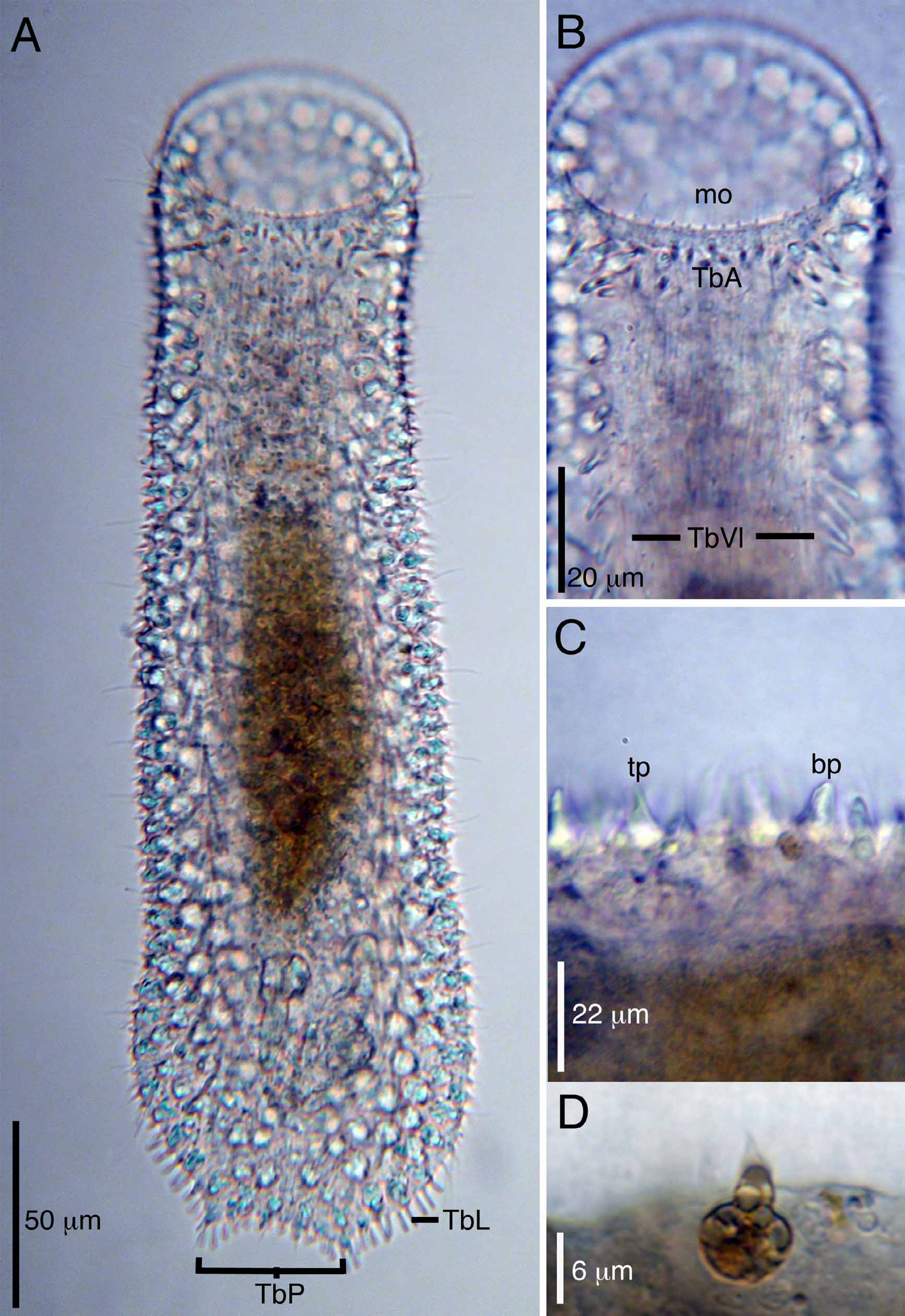
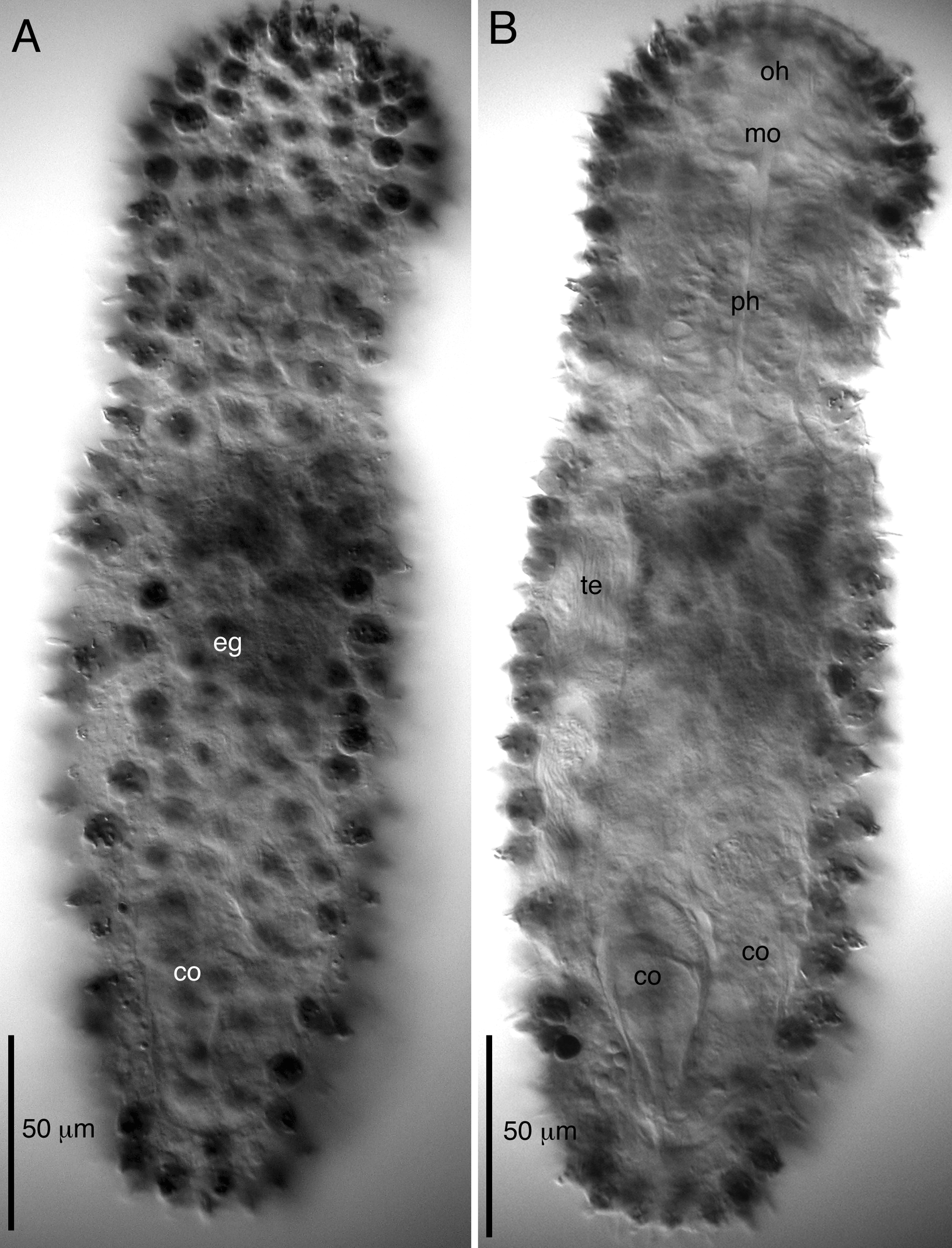
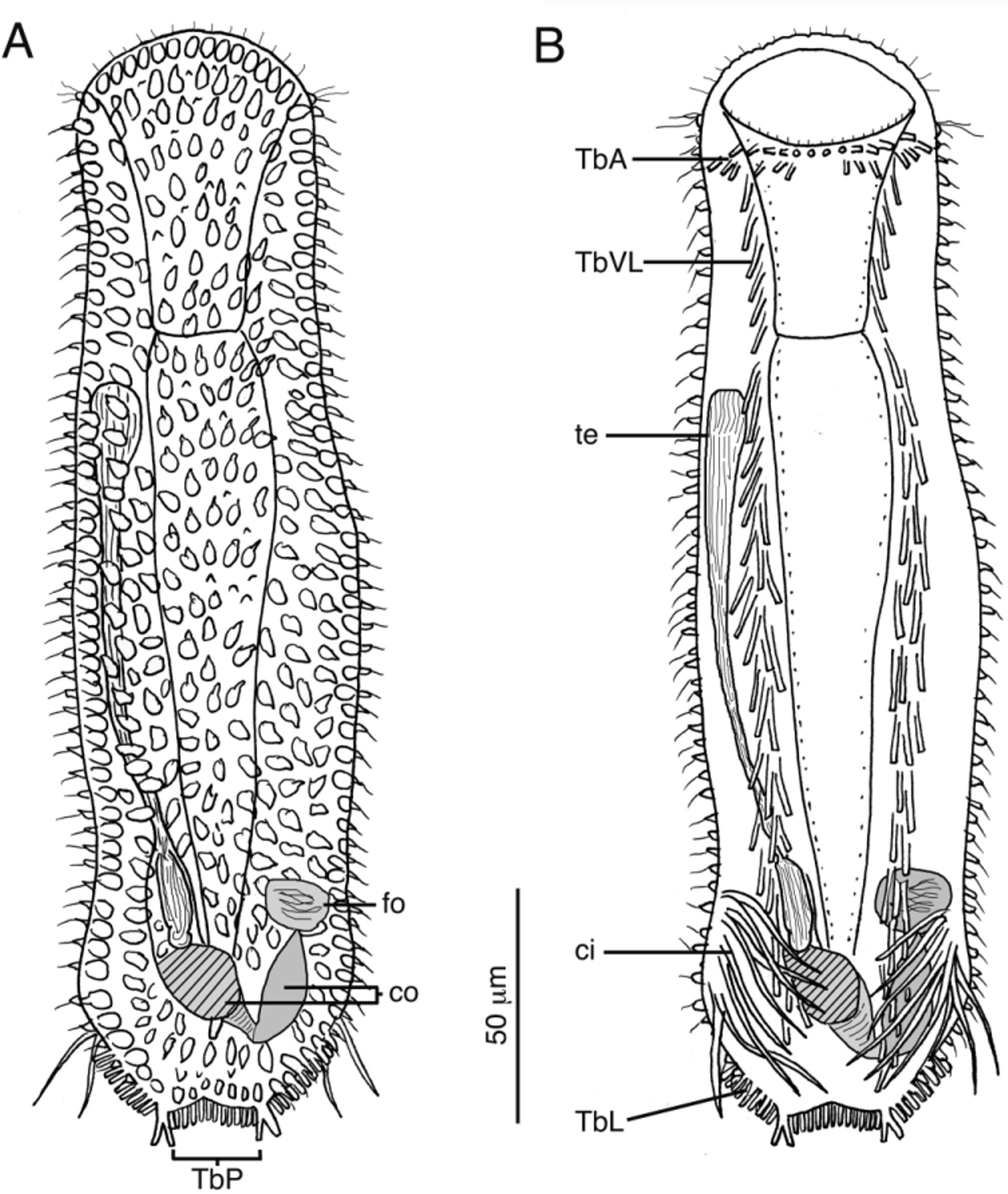
Diagnosis. Specimens with body lengths to 370 Μm long. Maximum body width at mouth/PIJ/midpoint of body is 65/87/95 Μm, respectively. Pharynx to 115 Μm long (measured from the tip of the oral hood); oral hood to 50 Μm long; mouth to 65 Μm wide. Ocelli absent. Cuticle is translucent and covered with blunt and triangular papillae on dorsal, lateral and ventrolateral surfaces. Numerous glandular cells fill the dorsal and lateral epidermis. Up to 16 TbA per side form two rows that cluster toward the lateral body margin, inserting directly on body surface. TbVL form a column of ca. 40 tubes down to pedicles, evenly spaced and sparse in the phayrngeal region, becoming more clustered in the trunk. TbL present just anterior to the caudal pedicles, to 10 per side, distinctly set off from TbVL and beginning at ca. U83, extending to caudum. TbP 2 per side, fused at their base forming pedicles, with 14 TbP between the pedicles. Ventral cirri present, 9 per side to 50 Μm long, in caudal region. Locomotor cilia form transverse rows on ventral surface from mouth margin to caudum. Hermaphroditic, with singular testes on right side as seen from above; egg dorsal at mid-body length; caudal organ is clearly bipartite with distinct muscular and non-muscular regions; frontal organ is sac-like.
Type material. The description of Oregodasys katharinae sp. nov. is taken from 12 specimens from station CBC10.11. All specimens were measured in vivo. Holotype ( USNM # 1146556) is ca. 312 Μm long, dorsoventrally oriented, with visible caudal organ.
Type locality. CBC10.11 is a deep outer slope at 42 m depth. CBC10.10 is a sand trough next to a ridge along the Carrie Bow Cay Reef at 30 m depth.
Etymology. The species is dedicated to friend and colleague, Dr. Katharina Jörger , who collected and helped to sort the new species.
Ecology. Few specimens present at CBC10.10, but moderately abundant at CBC10.11. Grain size analysis (Table 1) reveals a positive skewness, indicating that grain sizes larger than the median are predominant. Sediment with low amounts of organic detritus.
Description. Specimen length varied from 215 Μm (n=1, juvenile) to 370 long (adult range: 300 Μm–370 Μm, n=11). The type description is based mostly on one adult specimen of approximately 370 Μm total body length. All animals possess a highly glandular bodywall epithelium that is shiny under reflected light. Head has a glandular oral hood to 50 Μm long. Tip of hood with smooth margin and void of papillae. Numerous short, scattered cilia to 8 Μm long occur along the hood margin. Pharynx reaches 115 Μm in length (range: 82 Μm–115 Μm) to tip of oral hood. PIJ at U31. Pharyngeal pores not observed. Body width at mouth/neck/PIJ/ trunk are 72/70/74/90 Μm, respectively.
Body covering of two shapes of papillae, blunt and triangular, each ca. 7–13 Μm long; each triangular papilla is closely associated with a sensory cilium to 10 Μm long. Approximately 40 sensory cilia/side. Papillae scattered on dorsal surface but abundant on lateral and ventrolateral body surfaces. Triangular papillae are associated with an underlying epidermal gland to 8 Μm diameter; glands often brown in color and refractile with transmitted light. Blunt papillae may be associated with an underlying epidermal gland, but this was not always evident.
Adhesive tubes present in four series. Up to 16 anterior adhesive tubes (TbA) per side form two transverse rows beneath the ventral mouth margin. The more anterior row is formed of 8–10 tubes (ca. 8–10 Μm long), with the two medial tubes the shortest (ca. 6 Μm). A second transverse row is positioned posterior of the first row and contains 5–6 tubes (ca. 8–10 Μm long) that become clustered toward the lateral body margin. The ventrolateral adhesive tubes (TbVL) are positioned on either side of the ventral locomotory cilia and extend from approximately U17 to the caudal end. In the pharyngeal region, the TbVL form a singular column of six evenly spaced tubes, each ca. 12–15 Μm long. In the trunk, the TbVL are slightly more elongate (up to 20 Μm) and clustered; generally two tubes insert in proximity to one another along the length of the column. There are up to 30 tubes/side in the trunk region. A clustered set of lateral adhesive tubes (TbL), up to 7 Μm long and 10/ side, are set off from the TbVL at ca. U87, where they form a column that leads to the caudum. The caudum is formed of two pedicles, each bearing 2 posterior adhesive tubes (TbP). Each pedicle measures to 12 Μm in total length (including the tubes). Up to 14 adhesive tubes, to 8 Μm long, are positioned between the two pedicles.
Nine ventral, elongate cirri per side are present in the posterior trunk region, beginning at U77, and positioned lateral to the TbVL and anterior to the TbL. Each cirrus is curled medially and extends up to 50 Μm long.
The ventral ciliature forms a cotinuous series of transverse rows from the ventral mouth margin to the caudal pedicles. Cilia are 12–15 Μm long.
The digestive tract begins with a wide mouth, to 70 Μm, covered by an oral hood that extends to U14. The hood is covered with epidermal glands but appears free of papillae. The anterior margin of the hood, ca. 5 Μm, is free of glands. Numerous short cilia extend along the margin of the hood. The pharynx extends up to 115 Μm long and narrows to ca. 50 Μm at the PIJ around U41. The intestine is narrow and tapers toward the posterior end where it terminates in an anal pore at U89. A single juvenile specimen measuring 215 Μm long had the following set of measurements: mouth, 38 Μm wide; oral hood, 25 Μm long; and pharynx, 83 m long.
Animals are simultaneous hermaphrodites. A single testis is present on the animal’s right side (as observed from above) and extends from the PIJ to the caudal organ. Vas deferens opens into a muscular, upside-down, pear-shaped caudal organ on the right side of the body at ca. U72. The organ is clearly bipartite. The right half of the organ is 52 Μm long and 25 Μm wide. Distally, where the vas deferens enters into the caudal organ, the organ is bulbous (to 25 Μm wide) and wrapped in strong circular muscles. Caudally, the organ narrows to ca. 10 Μm but retains a circular muscle sheath. The other half of the organ is somewhat spindle-shaped (ca. 50 Μm long x 18 Μm wide) and devoid of musculature. Distally, the organ widens into a sac-shaped frontal organ (ca. 35 Μm diameter), devoid of musculature and containing spermatozoa (allosperm), which is in proximity to maturing ova. A single mature ovum was measured as 55 Μm long x 40 Μm wide in one adult specimen.
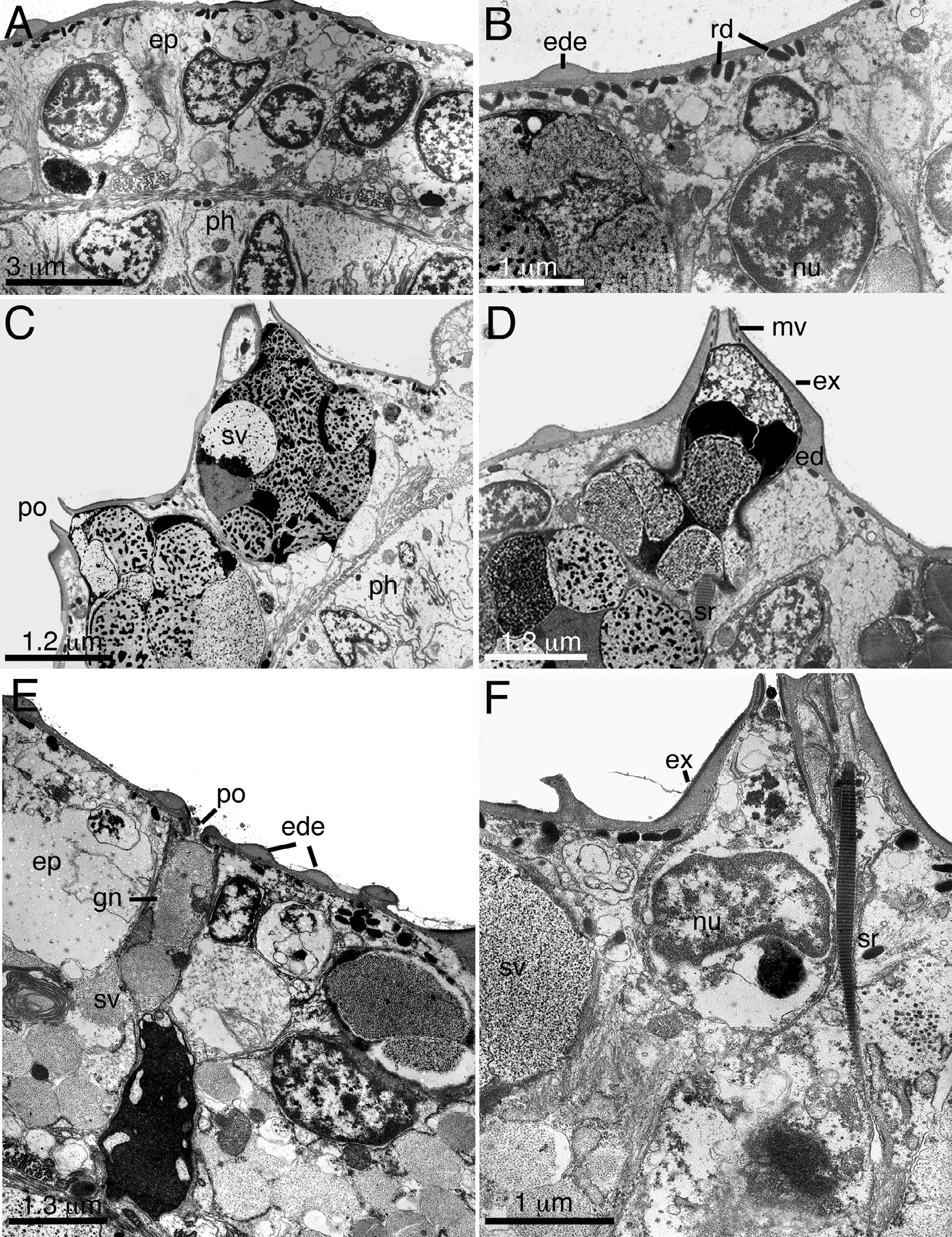
Ultrastructural Observations of O. katharinae sp. nov. The ultrastructure of the body wall of a single specimen was observed to document characteristics of the cuticle, epithelium and epidermal glandular system ( Fig. 6 View FIGURE 6 ). Externally, the monolayered epithelium ( Fig. 6 View FIGURE 6 A) is bound by two cuticular layers, a thin exocuticle ( Fig. 6 View FIGURE 6 D, F; also called the lamellar layer of Rieger & Rieger 1977) and thicker endocuticle (Fig. D, F; also called the basal layer of Rieger & Rieger 1977). The exocuticle appears to consist mostly of 1–2 electrondense sheaths, each ca. 10 nm in thickness, though fixation was relatively poor and it is difficult to distinguish whether the sheets are constructed as monolayers or bilayers (see Rieger & Rieger 1977 for explanation). The exocuticle lines the dorsal, lateral and ventral epidermis including the anterior adhesive tubes (TbA), ventral locomotory cilia, and sensory cilia. Cross sections through individual cilia revealed only 1–2 exocuticular sheaths on most locomotory cilia, and more than one sheath on some sensory cilia. Poor fixation made it difficult to determine the maximum number of exocuticular sheaths on sensory cilia or along the general body wall; however, in some regions of the body, the exocuticle was up to 53 nm thick, indicating multiple exocuticular sheaths.
The underlying endocuticle is 100–510 nm thick and homogeneous in appearance, displaying only a granular texture ( Fig. 6 View FIGURE 6 ). The endocuticle varies in thickness across a cross section of the body: endocuticle lining the ventral epidermis is ca. 100–240 nm thick; endocuticle lining lateral body wall is 100–250 nm thick; and endocuticle lining the dorsal epidermis is 140–510 nm thick. In general, most of the body is covered by a thin endocuticle from 100–200 nm thick, but the endocuticle thickens in regions around the dorsal and lateral papillae and where electron-dense elements are located. Endocuticle at the base of an individual triangular papilla may reach 460 nm in thickness, but it quickly thins out toward the apical end of the papilla and appears more similar to the endocuticle that lines the general body wall. Dorsally, there are several electron-dense elements up 326 nm thick and 950 nm long that are embedded within the endocuticle, making the total endocuticule up to 510 nm thick. These elements have a homogeneuos, electron-dense apperance and are distinguishable from the rest of the endocuticle by their unique bicorn hat shape ( Fig. 6 View FIGURE 6 B, D, E). The elements are not obviously associated with any papillae or sensory structures; however, serial sections were not examined.
The epidermis is cellular and monolayered. All cells are irregular in shape and contain a large nucleus, various-sized electron-lucent vesicles, few mitochondria, ER and Golgi ( Fig. 6 View FIGURE 6 A). Adhearens junctions anchored most cells to each another; septate junctions were more common in the dorsal epidermis than the ventral epidermis. The dorsal epidermis contained abundant electron dense, rod-shaped secretions to 170 nm x 240 nm, present just below the plasma membrane ( Fig. 6 View FIGURE 6 A, B). These secretions were present in all examined epidermal cells, but were less abundant in the lateral and ventral epidermis. Several dorsal epidermal cells contained dense tonofilaments that appeared to anchor the cells to the underlying basal lamina (not shown). These filaments were similar to filaments present in the anterior adhesive tubes, which are located on the ventral mouth margin (not shown). The adhesive tubes are duogland and contained at least one adhesive gland with large vesicles to 280 nm diameter (see Tyler & Rieger 1980 for detailed descriptions of the duogland system) and one releasing gland with small vesicles to 65 nm in diameter. The precise number of glands in each adhesive tube was not quantified because no cross sections were analyzed.
Scattered throughout the dorsal and dorsolateral epidermis were abundant epidermal gland cells (glandulocytes). Glandulocytes were associated with triangular papillae ( Fig. 6 View FIGURE 6 C, D) or insunk into the epidermis ( Fig. 6 View FIGURE 6 E). No sections were taken through the blunt-ended papillae. All gland cell perikarya are monocellular and flask shaped with a basal nucleus and voluminous secretory portion. Most glandulocytes were associated with a triangular papilla that extends above the epithelium and appears volcano-like in longitudinal section. The papilla itself is not formed of epidermal cells but instead is formed from an individual glandulocyte that is generally sandwiched between epidermal cells. The apical portion of the glandulocyte opens via a singular pore that is formed by microvillar extensions of the apical side of the glandulocyte ( Fig. 6 View FIGURE 6 D). The opening of the pore is lined by both exo- and endocuticle ( Fig. 6 View FIGURE 6 D). Membranebound secretion vesicles are present just below the pore ( Fig. 6 View FIGURE 6 C, D). The bulk of the glandulocyte perikaryon is taken up by numerous membrane-bound secretion vesicles, all of which show irregular staining. Secretory vesicles range from ca. 300 nm –2 Μm in diameter. Individual vesicles within a single glandulocyte may display a range of staining characteristics, including contents that appear as either electron dense, finelygranular, flocculent, or a range of all three within a single vesicle. Some vesicles are electron lucent but may contain wisps of flocculent material. There was no obvious difference between staining characteristics of vesicles in papillary versus insunk glandulocytes. The perikaryon of insunk glandulocytes was often positioned between and below the general epithelium, with the nucleus and other organelles pressed up against the basal lamina ( Fig. 6 View FIGURE 6 E). Each glandulocyte presented an apical neck that extended up to 5 Μm long between epidermal cells. The necks open to apical pores to 300 nm wide. No microvilli were observed at the apical end. The lip of the pore is formed from the bilayered cuticle, with both layers turning inward to line the pore. Electron-dense elements within the endocuticle were often, but not always, present in proximity to the apical pore of the insunk glandulocyte. In nearly all glandulocytes, most of the cellular organelles including the nucleus, mitochondria, ER and Golgi were compressed in the basal region of the glandulocyte. Several of the triangle-shaped papillae were in proximity to sensory cilia, and striated rootlets were regularly observed in cells adjacent to, but not necessarily part of, the papillae. One glandulocyte appeared to contain portions of a striated rootlet within the cell itself ( Fig. 6 View FIGURE 6 D); unfortunately, serial sections of the glandulocyte were not analyzed. Longitudinal muscles and neurons were present beneath some, but not all of the glandulocytes.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Thaumastodermatinae |
|
Genus |