Perisesarma maipoense ( Soh, 1978 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.5342798 |
|
persistent identifier |
https://treatment.plazi.org/id/03C187C1-1077-BC5F-FC40-8C24FED8FD01 |
|
treatment provided by |
Diego |
|
scientific name |
Perisesarma maipoense ( Soh, 1978 ) |
| status |
|
Perisesarma maipoense ( Soh, 1978) View in CoL
( Figs. 1–4 View Fig )
Chiromanthes maipoensis Soh, 1978: 11 View in CoL , Pls. 1a, d, 2c, 5a, b.
Sesarma (Perisesarma) maipoensis – Easton & Leung, 1993: 96; Lee & Leung, 1999: 65 Pl. 6.
Perisesarma maipoense View in CoL – Ng et al., 2008: 222.
Material examined. – 1 paratype female (20.0 x 15.4 mm) ( ZRC 1975.7.1.1), Mai Po marshes near Shum Chun river, new Territory ,
Hong Kong, coll. C. L. Soh, 15 Jun.1975. Others: 2 males (31.7 x 25.9 mm, 25.3 x 19.9 mm), 2 females (21.7 x 16.6 mm, 24.8 x 18.9 mm) ( ZRC), 6 males (30.1 x 25.0 mm, 29.1 x 23.5 mm, 23.6 x 18.9 mm, 24.9 x 19.5 mm, 22.9 x 18.0 mm, 18.7 x 14.4 mm), 1 ovigerous female (26.4 x 20.2 mm), 2 females (21.0 x 16.1 mm, 21.9 x 16.9 mm) ( ZMB), Con Ngan RAMSA site, Xuan Thuy, Nam Dinh Province, near bank of Red River estuary , Vietnam, coll. N. K. Hoang, 6 Jul.2008; 2 males (22.0 x 17.0 mm, 25.0 x 19.6 mm), sea bank close to Nam Phu Mangrove, Tien Hai District , Nam Dinh Province, near the red river estuary , coll. Do Van Nhuong, 18 Aug.2001; 2 males (30.2 x 24.8 mm, 27.6 x 22.0 mm) ( ZMB), 3 females (24.9 x 19.2 mm, 23.4 x 18.0 mm, 22.0 x 16.5 mm), Garden of Mangrove Ecosystem Research Station, Giao Lac commune, Giao Thuy District , Nam Dinh Province , coll. Do Van Nhuong, 27 Aug.2005.
Diagnosis. – Carapace 1.22–1.26 times broader than long; regions well defined with mesogastric and cardiac regions clearly demarcated; lateral carapace surface lined with strong oblique striae; carapace surface with sparsely scattered tufts of setae anteriorly, less setae posteriorly. Front 0.5 times carapace width, moderately deflexed with broad median emargination. Post frontal lobes prominent, median lobes slightly broader than lateral, separated by narrow, deep grooves. Anterolateral margin with sharp exorbital angle and single prominent epibranchial tooth. Posterolateral margin lined with rows of short setae.
Chelipeds subequal, large, robust. Merus with outer margin tuberculate, with subdistal spine; inner margin tuberculate, ending in large subdistal spine; outer surface with striations, inner surface with longitudinal row of setae. Carpus with inner angle not produced, outer margin and dorsal surface striated. Inner margin with row of granules, inner face with longitudinal row of granules. Palm without setae. Upper surface of palm with 2 short transverse pectinated crests distally. Primary (outer) crest composed of 15 or 16 pectinated teeth and 4 or 5 non-pectinated ones. Secondary (inner) crest well developed, shorter than primary, with 12 or 13 pectinated teeth and 6 or 7 non-pectinated ones. Outer surface of palm coarsely granulated, larger on median area, with distinct median longitudinal row of granules, smaller granules near upper margin; outer surface near lower margin striated proximally; indistinct ridge from mid length to tip of fixed finger near lower margin; inner surface of palm with several tubercles, large transverse tubercles near articulation with dactylus. Fixed finger slightly flattened laterally, distal two-thirds of outer surface of fixed finger almost smooth or with very low tubercles. Dorsal surface of dactylus with 5–8 flattened, indistinct tubercles, each tubercle with fine transverse lines; inner edge of dorsal border, behind main row of striated tubercles, with 12–14 relatively smaller conical granules which gradually become smaller distally. Fingers with tips chitinous, cutting edges with small and large teeth, leaving very wide gape when closed in adult males.
Walking legs robust, flattened, broad; second and third pairs subequal in length, longer than others, about 1.5 times carapace width. Merus of third leg approximately twice as long as wide; upper margin of merus with acute subdistal spine. Carpus with 2 carinae on outer surface. Propodi 2.4–2.7 times as long as wide, short stiff brushlike setae along dorsal margin, ventral margins with stiff setae distally. Dactylus about 0.8 times length of propodus, slightly recurved, terminating in acute calcareous tip, dorsal and ventral margins with long stiff setae.
Male abdomen relatively broad, all somites free. Telson semicircular, evenly rounded, slightly longer than somite 6; somite 6 about 1.8 times as long as wide, lateral margins slightly convex. Somites 3–5 progressively more trapezoidal, lateral margins of somites 4 and 5 straight, lateral margins of somite 3 convex, somite 2 very narrow laterally, larger medially, somite 1 very narrow longitudinally.
G1 slender, apical process long, bent to form an angle of about 45º, produced; corneous apex moderately long, tip rounded. Setae long, simple, originating at base of apical process and palp. G2 very short.
Female with smaller chelipeds, pectinated crests on palm replaced by 2 transverse rows of tubercles, dactylar tubercles indistinct.
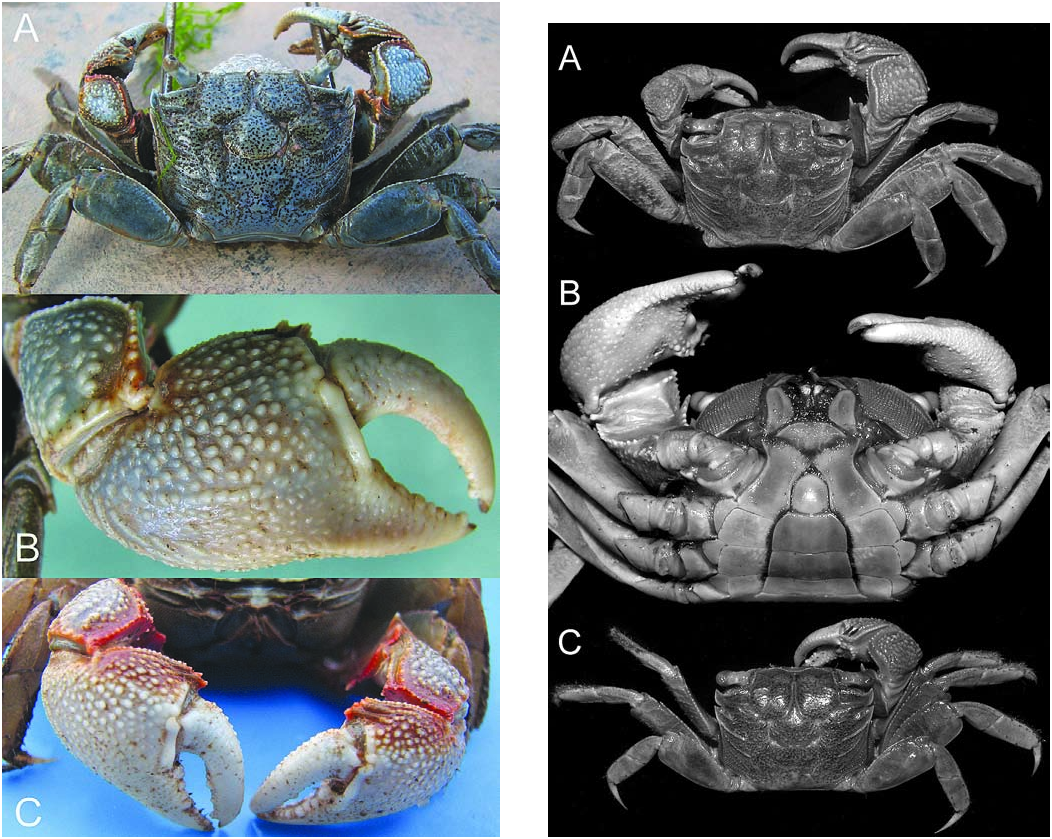
Colour. – Carapace whitish-blue to bluish-grey with scattered dark brown spots. Cheliped bluish-white, tubercles white, upper margin of carpus and merus reddish-brown with white tubercles. Ambulatory legs whitish-blue ( Fig. 1 View Fig ).
Remarks. – Soh (1978) described this species from one adult male measuring 27.0 × 21.0 (presently in British Museum, Natural History catalogue number 1976: 106) and one young male and one female from the Mai Po marshes near Shum Chun River in Hong Kong. One paratype female (20.0 × 15.4 mm, ZRC 1975.7.1.1) is in the ZRC. In general appearance, P. maipoense is closest to P. dussumieri (H. Milne Edwards, 1853) , as has been highlighted by Soh (1978: 12). Perisesarma maipoense , however, can easily be separated from P. dussumieri in having fewer and larger dactylar tubercles on the male chela (5–8 versus 11 or 12); the dactylar tubercles lack a chitinous peak (present on some tubercles in P. dussumieri ); the epibranchial tooth is relatively more acute and sharper; and the chitinous tip of the G1 is relatively longer and more strongly bent (versus a relatively shorter and straighter G1 chitinous tip of P. dussumieri ).
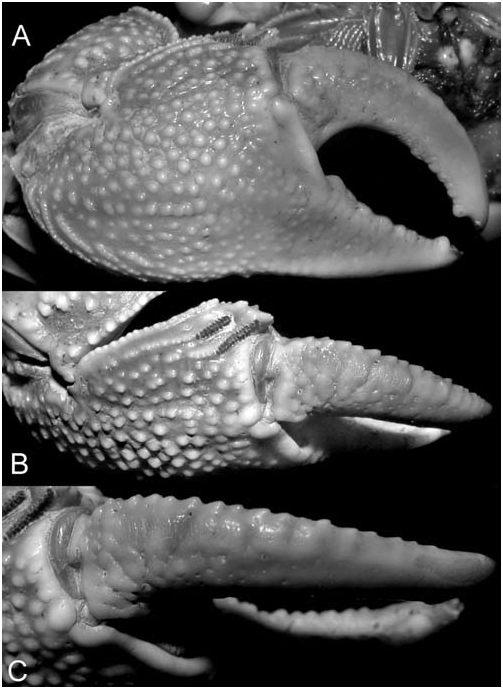
One of the most diagnostic features of P. maipoense is the small number of dactylar tubercles, each of which is very low and has shallow semicircular concentric ridges on it, none of which have a chitinous peak ( Fig. 3B, C View Fig ). The present specimens from Vietnam are interesting because although the smaller males (e.g., 25.3 × 19.9 mm, ZRC) agree well with the type description ( Fig. 3C View Fig ), the largest male (31.7 × 25.9 mm, ZRC), has 7 or 8 more prominent tubercles, although the distal ones are very low. The ridges on each dactylar tubercle are also more like uneven curved striae ( Fig. 3C View Fig ). With regards to the row of conical granules along the inner edge of the upper border of the dactylar finger, smaller males (e.g., ZMB: 22.9 × 18.0 mm, 18.7 × 14.4 mm) have the same number (12 or 13) as on the holotype male described by Soh (1978). However, larger males (e.g., ZMB: 30.1 × 25.0 mm, 29.1 × 23.5 mm, 24.9 × 19.5 mm; ZRC: 31.7 × 25.9 mm), have 14 to 16 conical granules of which the distal eight or nine have small chitinous caps. In both cases, this row of conical granules runs parallel to the main row of low striated tubercles, ending just before the distal quarter .
The carapace proportions vary according to size and sex. Larger males (e.g., ZMB: 30.1 × 25.0 mm, 29.1 × 23.5 mm, 24.9 × 19.5 mm; ZRC: 31.7 × 25.9 mm) are proportionately narrower, with the width to length ratios ranging from 1.20–1.23 ( Fig. 2A). Smaller males (e.g., ZMB: 22.9 × 18.0 mm, 18.7 × 14.4 mm; ZRC: 25.3 × 19.9 mm) are relatively broader with the width to length ratios ranging from 1.25–1.30 ( Fig. 2C); and adult females are consistently broader, being 1.29–1.33 times wider than long.
Soh (1978: 12) described the colour of his specimens as follows: “Carapace greyish brown and punctated with tiny dark spots. Walking legs grey brown. Upper border of palm and the edges of carpus of the chelipeds tinted with discontinuous dark red. Outer and inner surface of palm of chelipeds greyish brown. Fingers and telson yellowish white.” The present specimens agree well with this distinctive colour and pattern ( Fig. 1 View Fig ). In larger males, however, the dark red parts of the cheliped become more maroon and duller in colour ( Fig. 1A View Fig ). In addition, the outer surface of the chela can be a dirty-white ( Fig. 1B View Fig ).
Ecology. – Soh (1978: 12) stated that he obtained his specimens from “Burrows in muddy substratum near the water edge at Mai Po marshes”. Lee & Leung (1999) commented that in Mai Po, the species was only found on the raised levees of drainage channels and drier bunds near the tidal limit in mangrove areas, and is absent on the landward side of the border fence road. The present specimens from northern Vietnam burrow in the mangrove littoral zone, in areas both above and close to the average tide level. The crabs are amphibious and occur mainly in brackish water areas. They always live at the edge of the mangroves and have been observed inside the mangrove forest itself. The crabs have been obtained from different habitats. In the garden of the Mangrove Ecosystem Research Station at Giao Thuy District, the soil is relatively hard but the crabs nevertheless make burrows in the dry substrate. They also occur at the base of the sea-dyke slope where there is firm mud and grass growing (at Xuan Thuy); as well as in areas with wetter and muddier substrates near the water’s edge.
The burrows of P. maipoense are typically 30 to 45 cm deep, in harder ground, but from 35 to 50 cm deep in softer, muddier soil. It is relatively simple, without many chambers or side tunnels. Interestingly, like in some species of fiddler crabs ( Ocypodidae : Uca ), the entrance to their burrows may be raised by 2 or 3 cm above the surface like a chimney. Specimens are difficult to catch because they are extremely wary of humans. They are caught mainly using fishing lines with baited hooks; or at night by the water’s edge using head torches.
Studies by Poovachiranon (1986) and Lee (1993) in Hong Kong have shown that P. maipoense is mainly a herbivore, feeding on dead leaves (see also Lee & Leung, 1999); and this is also the case for the Vietnamese populations. Analysis of their gut contents showed that their food includes small leaves and grass. They have been observed pulling pieces of vegetation into their burrows. The crabs forage day and night.
It is not clear if there is any distinct breeding season, although relatively more ovigerous female crabs have only been observed during summer (March to July). In winter, the crabs are not active and tend to stay in their burrows.
Distribution. – Perisesarma maipoense has been regarded as a Hong Kong and Macau endemic since its discovery (see Lee, 1995; Lee & Leung, 1999; Anonymous, 2008, 2009). Lee & Leung (1999: 65) noted that “ P. maipoensis has a very restricted geographic distribution even within Hong Kong. It has been reported from Tung Chung on Lantau Island and Futian on the northern shores of Deep Bay, and from Macau (Easton & Leung 1993).” Recent studies of Hong Kong and southern Chinese sesarmids (e.g., Kwok et al., 2005) did not encounter the species and Anonymous (2009: 5) commented that the “species has not been recorded [from Hong Kong] within the last 10 years”. The present record extends its range to northern Vietnam.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Perisesarma maipoense ( Soh, 1978 )
| Ng, Peter K. L., Khac, Hoang Ngoc & Rahayu, Dwi Listyo 2010 |
Perisesarma maipoense
| Ng, P 2008: 222 |
Chiromanthes maipoensis
| Soh, C 1978: 11 |