Paranaxia serpulifera
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4127.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:6B17FBC8-E20D-480F-9C81-112700AEB0FA |
|
DOI |
https://doi.org/10.5281/zenodo.5631928 |
|
persistent identifier |
https://treatment.plazi.org/id/D12E8B41-FFB9-FFE9-FF32-F9E0FBE2E492 |
|
treatment provided by |
Plazi |
|
scientific name |
Paranaxia serpulifera |
| status |
|
Paranaxia serpulifera (Guérin, 1832, in Guérin-Méneville 1829 –1837)
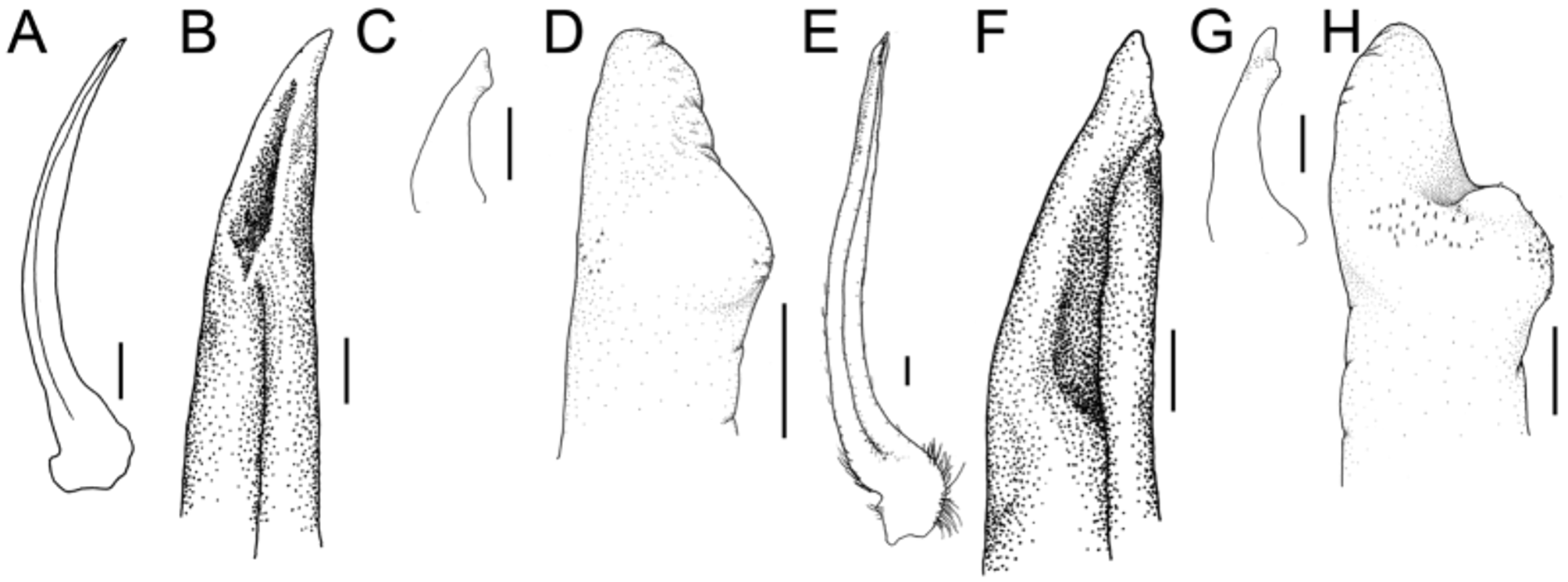
( Figs 1 View FIGURE 1 , 3A–D View FIGURE 3. A – D , 4 View FIGURE 4 A–D)
Pisa serpulifera Guérin, 1832 , in Guérin-Méneville 1829 –1837: 9, pl. VIII, fig. 2-2d.
Naxia View in CoL serpulifera— H. Milne Edwards 1834: 313.— Miers 1879: 658.— Haswell 1882: 21.— Miers 1884: 196.
Naxioides View in CoL serpulifera— Rathbun 1897: 157; 1914, 661, pl. 2, figs 9, 10.
Paranaxia serpulifera— Rathbun 1924: 7.— Montgomery 1931: 417.— Griffin 1966, 266, pl. 17, fig. 2a.— Balss 1935: 127.— Sakai 1976: 264, fig. 143.— Griffin & Tranter 1986: 280, fig. 103.— Morgan 1987: 337, figs 1, 2.— Jones 1990: 193. — Poore 2004: 384, fig. 117g, h.— Low et al. 2013: 117, table 2.
Non Paranaxia serpulifera— Rahayu & Ng 2000: 889 [= Paranaxia keesingi sp. nov.].
Material examined. Lectotype herein designated: MNHN-IU-2000-4479, 1 female, 86.2x 68.2 mm, “Nouvelle Hollande ”.
WESTERN AUSTRALIA: WAM C5640, 1 male, 48.0 × 36.1 mm, Cottesloe, 13 Jul. 1939, L. Glauert coll.
WAM C5904, 1 female, 99.5 × 79.7 mm, 40 km north of Geraldton, 12 Mar. 1941, A.S. Carr coll. WAM C5907, 1 female, 71.6 × 56.2 mm, Mandurah, 14 Apr. 1941, W.J. Budges coll. WAM C6043, 1 female, 90.3 × 69.5 mm, South Channel, Hudson Naval Base, 22 Dec. 1944, J.H. Wallett coll. WAM C6675, 1 female, 48.7 × 37.0 mm, Broome, 17°58`S 122°14`E, 6 Mar. 1951, S. Hamilton coll. WAM C7345, 1 male, 74.7 × 59.9 mm, Mandurah, 4 Jun. 1957, R. Chalmers coll. WAM C14026, 1 male, 83.0 × 63.3 mm, South Mole, Fremantle, 32°03`00"S 115°44`00"E, Jan. 1959, W.F. Hawkins coll. WAM C16001, 1 female, 97.8 × 77.2 mm, Sunday I., Shark Bay, 14 Mar. 1986, G.J. Morgan coll. WAM C16002, 1 female, 88.7 × 68.9 mm, Sunday I., Shark Bay, 14 Mar. 1986, G.J. Morgan coll. WAM C16619, 1 female, 91.1 × 71.7 mm, Cockburn Sound, Jan. 1986, R. Beale coll. WAM C34792, 1 male, 76.9 × 61.2 mm, Shark Bay, 25°36.99`S 113°14.45`E, 15.7 m, 29 Jun. 2006, S. Morrison et al. coll. WAM C34793, 1 female 52.5 × 37.8 mm, Shark Bay, 25°55.50`S 113°14.02`E, 12.8 m, 24 Jun. 2006, S. Morrison et al. coll. WAM C38296, 1 male, 60.0 × 44.8 mm, Point Cloates, on beach, 22°43`S 113°40`E, 7 Oct. 1957. WAM C38363, 1 male 78.6 × 63.2 mm, 1 female, 68.0 × 53.4 mm, Giralia Bay, Exmouth Gulf, 22°27`S 114°20`E, 31 Mar. 1990, D.S. Jones coll. WAM C41265, 1 male, 62.4 × 42.2 mm, Admiralty Gulf, Kimberley, 14°16`S 125°52`E, 4 m, 23 Feb. 1968, E.H. Barker coll. WAM C41267, 1 female, 104.5 × 79.7 mm, Walsh Point, Admiralty Gulf, Kimberley, intertidal, 10 Aug. 1978, F.E. Wells coll. WAM C54847, 1 female, 104.9 × 83.6 mm, Green Is, 30°40`55"S 115°06`23"E, 26 Apr. 1959, R.W. George coll. WAM C54870, 1 female, 113.5 × 91.8 mm, Sandy Cape, 30°11`S 114°59`E, 2 Jan. 1960, E.P. Hodgkin & L.M. Marsh coll. WAM C55209, 1 male, 85.9 × 69.2, 1 female, 99.3 × 78.8 mm, Denham Sound, 25°46`S 113°15`E, 3 Mar. 2004. WAM C55210, 1 male, 99.6 × 73.4 mm, 1 female, 93.4 × 72.8 mm, Denham Sound, 25°46`S 113°15`E, 3 Mar. 2004. WAM C55211, 1 male, 91.3 × 72.9 mm, Jurien Bay, 30°15`S 115°01`E. WAM C55212, 1 male, 88.6 × 72.0 mm, Woodman Point, Cockburn Sound, 32°08`S 115°44`E, 10 Apr. 2013. WAM C55478, 1 male, 51.5 × 36.9 mm. WAM C55479, 1 male, 79.1 × 61.4 mm, Shark Bay, 25°32.90`S 113°13.25`E, 19.3– 18.7 m, 29 Sep. 2003, S. Morrison & P. Unsworth coll. WAM C61241, 1 male, SOL56, W of Heywood Island, Bonaparte Archipelago, 15°22`35.53"S 124°11`33.98"E to 15°22`34.31"S 124°11`31.46"E, 35 m, 19 Mar. 2015, coll. J. Fromont & L. Kirkendale coll. RV Solander. WAM C59957, 1 male, PMCP /051, off Abutilon Island, Pilbara, 20°44`22"S 115°35`58"E, 12– 8.7 m, 22 Jun. 2013, E. Morello, G. Fry, M. Miller, D. Thomson, & D. Bearham, coll. RV Naturaliste.
Diagnosis. Body, pereiopods completely covered in dense pubescence, hooked setae present on rostrum, preorbital spines, anterolateral carapace margins, dorsal tubercles, all ambulatory leg articles, except dactyls. Rostral length 0.1–0.45 times CW. Subhepatic spine large, directed anteroventrally. Pterygostomian region unarmed. Intestinal tubercle rarely produced beyond posterior carapace margin. Anteriormost sternal pit reniform, deep, well defined in both sexes; paired lateral sternal pits shallow poorly defined relative to anterior pit in males, absent in mature females. Chelipeds of mature males (> 80 mm CW) elongate, merus extends beyond rostral apices, prominent posterodistal tubercle blunt; propodus length up to 1.32 times CW. Pereiopods 2–5 stout, pereiopod 2 (P2) dactyl up to 0.48 times CW.
Description. Males. Carapace pyriform, PCL 1.23–1.50 times CW, regions well defined, densely covered in short pubescence; hooked setae present on tubercle apices, anterior branchial, hepatic margins. Rostral horns parallel, distally bifid, 0.24–0.50 times CW; tufts of hooked setae on lateral, dorsal, mesial surfaces. Orbits closed dorsally, ventrally; preorbital angle produced into large anterodorsally directed spine with hooked setae, postorbital lobe anteriorly cupped, dorsal orbital hiatus a narrow fissure. Gastric region elevated, slightly higher than other regions; 13 small tubercles distributed in anterior gastric region; apex with 3 large, blunt tubercles.
Hepatic region inflated, with acute stout spine on subhepatic margin directed anteroventrally, approximately same size as preorbital, branchial spines, visible in dorsal view.
Pterygostomian region unarmed. Branchial region inflated with 2 low mesial tubercles, lateral margin with large laterally directed spine above pereiopod 3, apex rounded. Cardiac region elevated, apex bluntly rounded. Intestinal tubercle large, apex rounded.
Eyestalks sparsely setose anteriorly, cornea terminal, retractable into orbit. Antenna basal article laterally expanded to form suborbital floor; flagellum inserted ventral to rostrum, not visible dorsally.
Maxilliped 3 with dense pubescence, thicker, longer along borders of articles; ischium narrower than merus, mesial margin dentate, lateral margin approximately 0.7 mesial margin length, outer surface with shallow longitudinal depression; merus subtriangular, anterolateral angle produced.
Chelipeds equal, becoming elongate in males> 80 mm CW, length up to 1.23 P2, merus extending beyond apices of rostrum, medial dorsal tubercle, blunt posterodistal tubercle; carpus smooth about as long as dactyl; propodus length 2.62–3.91 times height, 0.5–1.32 CW; smooth; dactyl with prominent tooth in gape, occludent margins of fingers crenulate.
Ambulatory legs stout, unarmed, covered in dense pubescence except at distal extremities of dactyls, hooked setae present dorsally on all articles except dactyls. P2 longest, 1.4 4–2.62 times CW. Dactyls evenly curved, P2 dactyl 0.28–0.48 times CW, unarmed on ventral surface.
Sternum with series of prominent pits surrounding pleon, each pit placed across junction of sternal segments, anterior-most pit on sternite 3, 4 reniform, posterior margin concave; 8 pits present adjacent to articulation with each pereiopod, sternite 8 pit smallest, all shallower than anterior-most pit. Pleonal-locking tubercles prominent, on sternite 5 near suture with sternite 4.
Pleon with 6 free somites plus telson, widest at somites 2, 3, somite 6 lateral margins convex, width 1.2 times length, longer than somite 5; telson triangular, width 1.25 times length, apex rounded. Raised ridge running length of pleon, all pleonal somites with transverse ridge, giving slight, paired lateral depressions present at junctions of each somite.
Gonopod 1 slightly curved laterally, tapering distally into an acute point, aperture subapical, basally with very short setae on lateral margins. Gonopod 2 stout, curved laterally, apex inflated, fabiform, with minute scattered subapical, stout setae. Penis coxal emerging from P5.
Females. Surface details of carapace, pereiopods 2–5 same as males. Subhepatic spine not always visible in dorsal view. Chelipeds much smaller, slender than males, propodus length 2.39–3.64 times height, 0.17–0.57 times CW, merus with blunt posterodistal tubercle. P2 1.30–2.05 times CW, dactyl 0.18–0.40 times CW. Sternal cavity on sternite 3, 4 same as male; lacking cavities on sternites 5–8. Pleon covering whole of sternum except sternites 1–3; broadest at somites 5, 6, telson short, broad width 3.0 length. Vulvae ovate, narrowing laterally, positioned in anterior half of sternite 6 submedially, blocked internally by chitinous vulvar valve.
Distribution. Australia: Western Australia (south to Perth), Northern Territory, Queensland. Depth: 0– 20 m.
Remarks. The specimen herein designated as lectotype (MNHN-IU-2000-4479) is presumed to be the individual figured by Guérin ( Fig. 1 View FIGURE 1 A–D). This specimen was labelled simply as “ Naxia serpulifera Milne Edwards Nouvelle Hollande ”. Although no dates are on the label, Nouvelle Hollande was no longer the official name of the Australian continent after 1824, suggesting that the specimen pre-dates Guérin’s publication. The female measures 116.7 mm CL, 86.2 mm PCL and 68.2 mm CW, which roughly corresponds to H. Milne Edwards’ measurements of “ 4 inches ”. The specimen agrees with the original figures as well as the females examined during this study and is in good condition except in that it is missing the pleon. Many of the specimens figured by Guérin, were deposited in the Academy of Natural Sciences of Philadelphia, but no specimens of Paranaxia serpulifera are listed in the catalogues of Spamer & Bogan (1992; 1994), further supporting the present designation.
Holthuis & Manning (1990) and Low et al. (2013) have shown that Guérin’s plate 8 was published no later than February 1832 and we have followed Low et al. ’s recommendation in citing 1832 as the publication date. Guérin (1832, in Guérin-Méneville 1829 –1837), however, credited H. Milne Edwards with the authority of Pisa serpulifera , but H. Milne Edwards’ description (under the name Naxia serpulifera ) was not published until 1834.
Our attribution of P. serpulifera to the specimens described by H. Milne Edwards (1834) is supported by his description of the size of the pterygostomian spine and comparison of the length of the ambulatory legs. He mentioned the presence of a spine on the pterygostomian region, of a similar size as the branchial and preorbital spines. The positioning of this spine is not shown in Guérin’s figures (Guerin 1832: Figs 1 View FIGURE 1 A, B), but can be clearly seen above the suture in the newly designated lectotype. This spine is always found above the suture line between the pterygostomian and the subhepatic regions in the examined specimens of P. serpulifera . In contrast, the spine is found below this suture and is clearly smaller than the branchial and preorbital spines in P. keesingi sp. nov. No subsequent description of P. serpulifera even mentions this spine. In relation to the legs, H. Milne Edwards describes the first pair being longer than the second in males (shorter in females). The chelipeds of male P. keesingi sp. nov., however, are always shorter than P2 ( Table 2 View TABLE 2 ), suggesting that H. Milne Edwards did not have material of both species available.
Paranaxia serpulifera is found in shallow water in northern Australia, often in seagrass beds and generally not on reefs. Despite its large size and prevalence, little research has been carried out on this species. The only nontaxonomic works have focused on the direct development and brooding of the young ( Rathbun 1914, 1924; Morgan 1987).
The record from Japan by Sakai (1976) bears the large subhepatic spine characteristic of P. serpulifera , but the presence of this species in Japan was considered doubtful by Griffin & Tranter (1986) and no subsequent records exist. The specimens recorded by Rahayu & Ng (2000) are referred to the newly described species (see below) and the authors consider P. serpulifera to be endemic to northern and western Australia.
TABLE 2. Summary of key morphological characters used to differentiate Paranaxia keesingi sp. nov. from P. serpulifera (Guérin, 1832, in Guérin-Méneville 1829 – 1837).
| P. serpulifera | P. keesingi sp. nov. | |
|---|---|---|
| Anterolateral spine placement | Subhepatic region | Pterygostomian region |
| Cheliped merus posterodistal spine | Low, blunt | Prominent, sharp |
| Male cheliped/ pereiopod 2 length | Up to 1.23 | Up to 0.70 |
| Paired sternal cavities | Relatively shallow | Deep, clearly demarcated |
| Male G1 | Evenly curved throughout | Curvature lessening distally |
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
SuperFamily |
Majoidea |
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
SuperFamily |
Majoidea |
|
Family |
|
|
Genus |
Paranaxia serpulifera
| Hosie, Andrew M. & Hara, Ana 2016 |
Paranaxia
| Rahayu 2000: 889 |
Paranaxia
| Low 2013: 117 |
| Poore 2004: 384 |
| Jones 1990: 193 |
| Morgan 1987: 337 |
| Griffin 1986: 280 |
| Sakai 1976: 264 |
| Balss 1935: 127 |
| Montgomery 1931: 417 |
| Rathbun 1924: 7 |
Naxioides
| Rathbun 1897: 157 |
Naxia
| Miers 1884: 196 |
| Haswell 1882: 21 |
| Miers 1879: 658 |
| Milne 1834: 313 |