Trilobatus trilobus ( Reuss, 1850 )
|
publication ID |
https://doi.org/10.1080/14772019.2019.1578831 |
|
DOI |
https://doi.org/10.5281/zenodo.10932447 |
|
persistent identifier |
https://treatment.plazi.org/id/072AAD72-2332-AE7F-3BDC-FACCFE57F2AA |
|
treatment provided by |
Felipe (2024-03-26 20:12:52, last updated 2024-04-05 13:37:31) |
|
scientific name |
Trilobatus trilobus ( Reuss, 1850 ) |
| status |
|
Trilobatus trilobus ( Reuss, 1850) View in CoL
( Figs 6A–O View Figure 6 , 16E, 17A, E View Figure 17 )
1850 Globigerina triloba Reuss : 374, pl. 47, fig. 11a–e.
1957 Globigerinoides triloba triloba (Reuss) ; Bolli: 112, pl. 25, fig. 2a–c.
1960 Globigerinoides triloba triloba (Reuss) ; Jenkins: 353, pl. 2, fig. 5a–c.
1966 Globigerinoides trilobus trilobus (Reuss) ; Jenkins: 9, pl. 2, fig. 8a–c.
1967 Globigerinoides quadrilobatus trilobus (Reuss) Closs : 340, pl. 1, fig. 22.
1975 Globigerinoides quadrilobatus trilobus (Reuss) Srinivasan : 139, pl. 2, fig. 7.
1983 Globigerinoides triloba (Reuss) Kennett & Srinivasan : 62, pl. 13, figs 1–3.
1994 Globigerinoides trilobus (Reuss) Loeblich & Tappan : 107, pl. 206, figs 1–6.
2012 Globigerinoides trilobus (Reuss) Rögl : 181, pl. 1, figs 1–7.
2018 Trilobatus trilobus (Reuss) Spezzaferri, Olsson, & Hemleben : 300–302, pl. 9.14, figs 1–21.
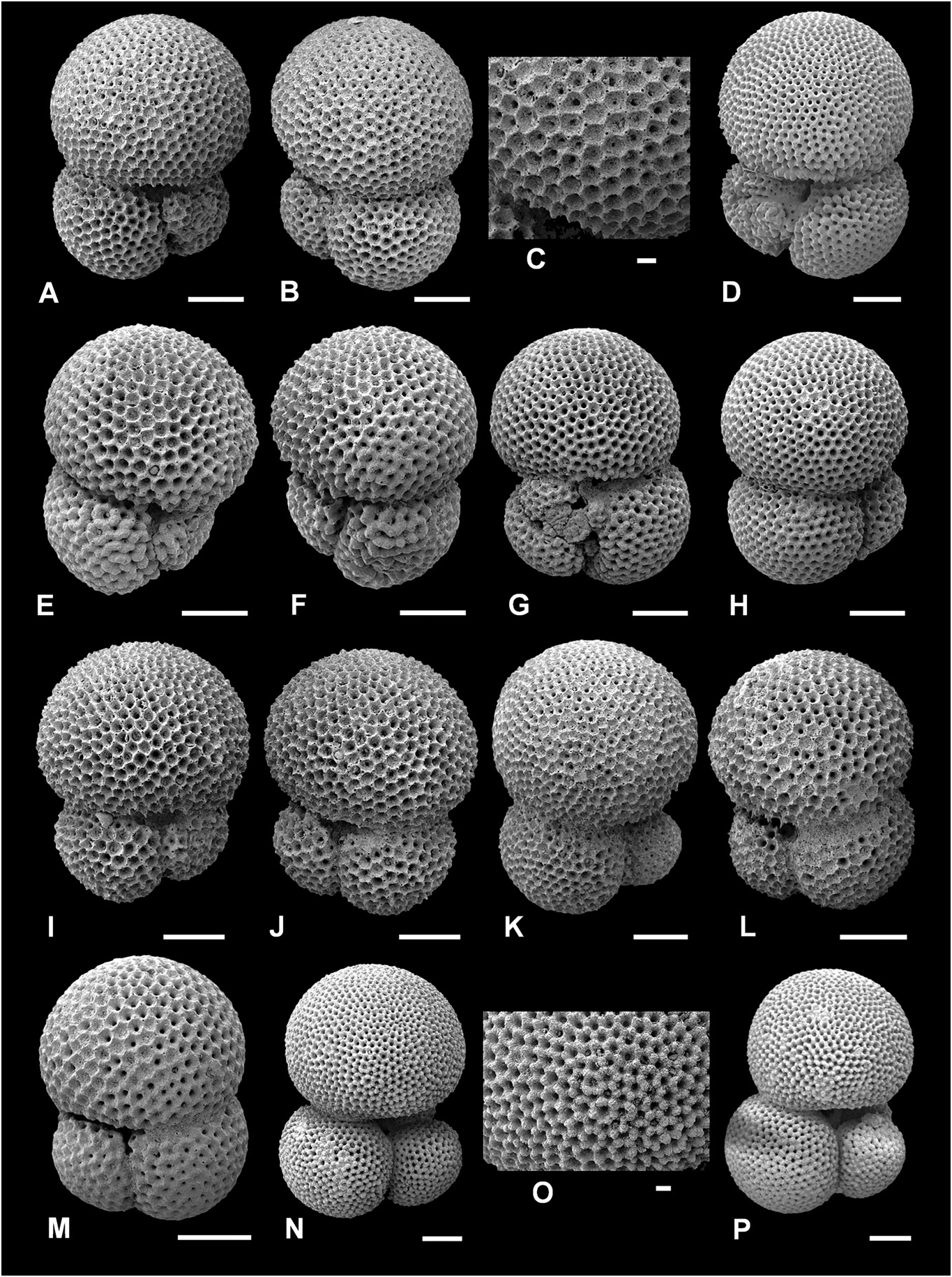
Description. Type of wall: spinose, coarsely cancellate (often termed polygonal or honeycomb) ‘ sacculifer - type’ wall texture. Test morphology: low trochospire, initially involute, later more evolute coiling, coiling direction random, three globose, near-spherical chambers in the final whorl, increasing rapidly in size as added, final chamber larger than all other chambers combined; sutures distinct, depressed, straight to slightly curved on both sides; umbilicus narrow; primary aperture interiomarginal-umbilical, a low arch, slit-like, no bordering rim; supplementary apertures small, placed at the sutures of the preceding chamber and third-previous chamber, often only one visible due to infilling or secondary calcification.
Note: description is based on the original description and species concept of Reuss (1850, p. 374), and also Kennett & Srinivasan (1983, p. 62) and Rogl (2012, p. 182), but is here emended and extended.
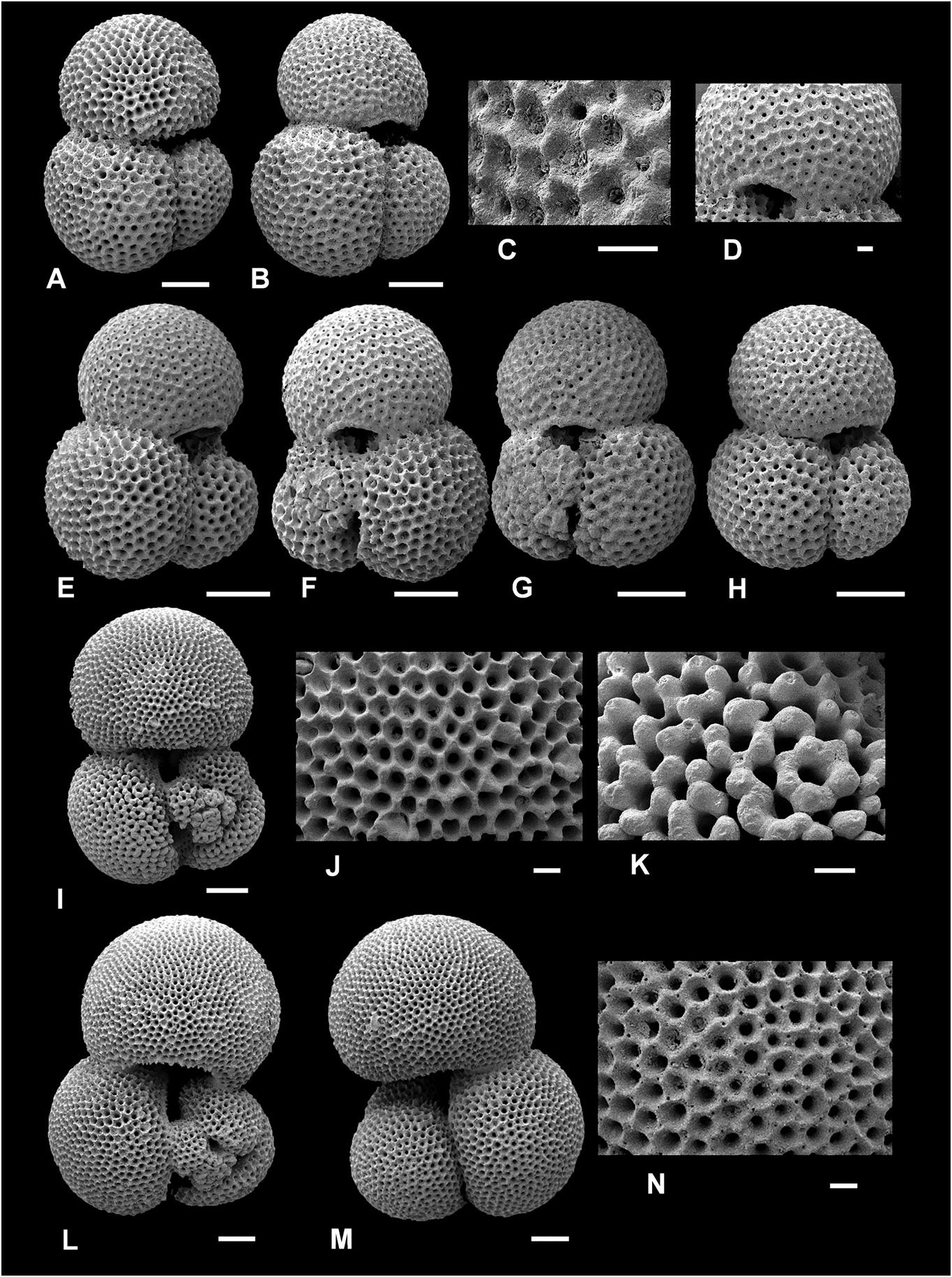
Remarks. Trilobatus trilobus is distinguished from T. immaturus and T. quadrilobatus by having more rapidly enlarging chambers in the final whorl, resulting in a final chamber that is larger than all other chambers combined. The last chamber is more embracing of the earlier chambers owing to tighter coiling, whereas T. immaturus and T. quadrilobatus are more loosely coiled with generally more than three chambers in the final whorl (3.5 to 4). Trilobatus trilobus differs from T. sacculifer which possesses a sac-like final chamber and from G. fistulosa which has protuberances. Globigerinoides altiaperturus Bolli, 1957 differs primarily in possessing a high-arched, semi-circular primary aperture, whereas in T. trilobus it is low and slit-like.
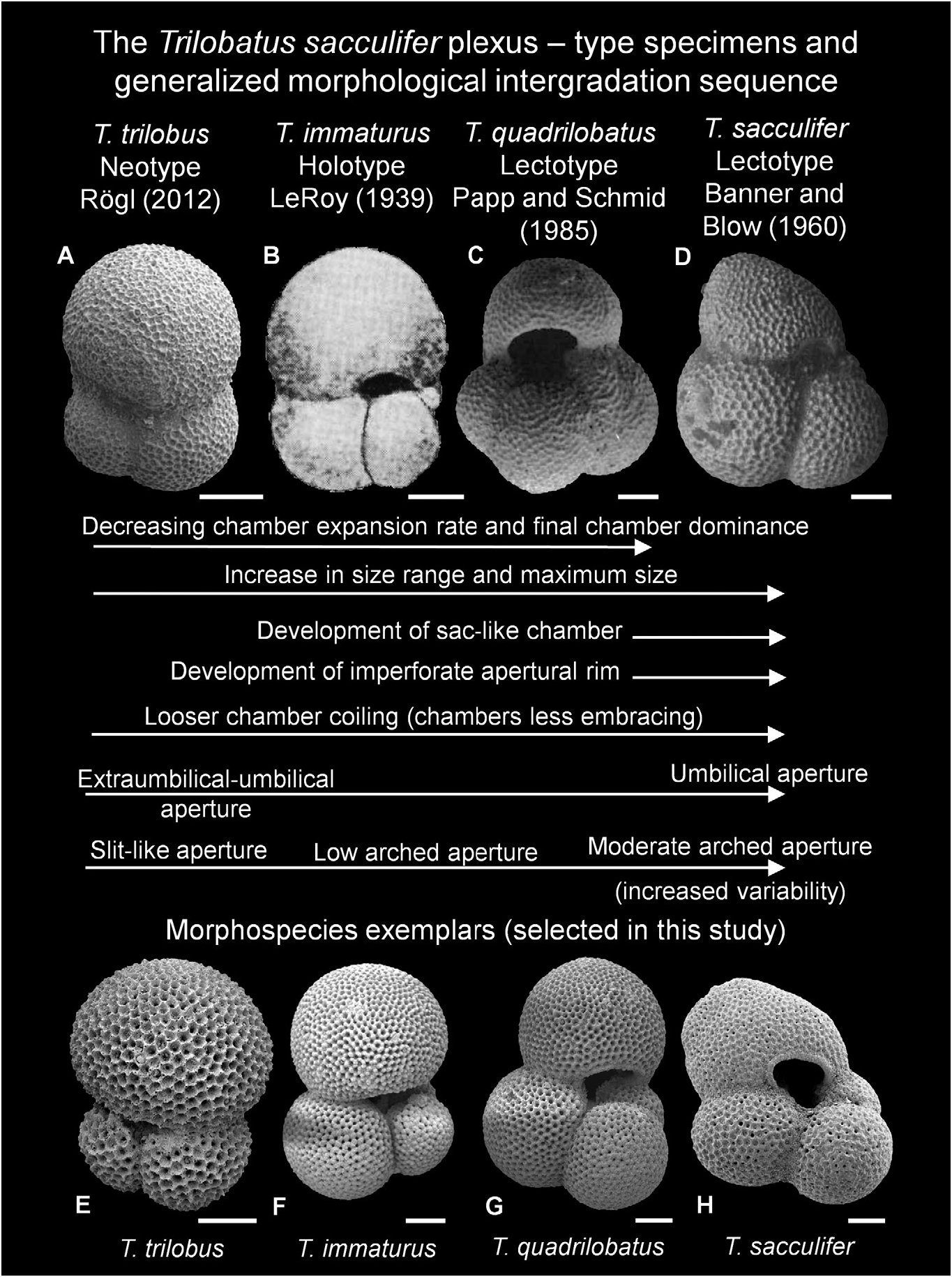
Type locality. Reuss (1850, p. 374) first described Globigerina triloba , documenting its occurrence in five separate localities from four countries ( Romania, Poland, Austria [two localities] and Italy; see also Rogl 2012, p. 181 for details). Rogl (2012) illustrated numerous specimens from these localities and designated a neotype (here reproduced in Fig. 17 View Figure 17 ) from the Polish locality material of Reuss (1867). Therefore, the type locality is the salt mine Wieliczka, near Krakow, Poland.
Taxonomic history. Reuss (1850, p. 374) described the new species Globigerina triloba and illustrated a specimen that clearly exhibits multiple apertures (pl. 47, fig. 11a–e). Reuss (1850) also highlighted how the last chamber is larger than the preceding chambers. The sutures are deeply incised, giving this morphospecies a distinct ‘three-lobed’ appearance, which explains the derivation of the name trilobus . After the introduction of Globigerinoides Cushman, 1927 , T. trilobus was accordingly assigned to this genus by later workers (see synonymy list).
Bolli (1957, p. 113) named Globigerinoides triloba altiapertura (= Globigerinoides altiaperturus ) as a subspecies for forms similar to T. trilobus and T. immaturus morphotypes, but possessing a higher arched, semi-circular primary aperture (see also Bolli & Saunders 1985, p. 192). However, G. altiaperturus is probably not closely related to T. trilobus (see Kennett & Srinivasan 1983, text-fig. 9; and Spezzaferri et al. 2015, fig. 5). Whilst G. altiaperturus is included in the above distinguishing features section, these morphospecies should not be confused morphologically due to their contrasting primary aperture morphology and wall texture.
Trilobatus trilobus has the most conservative morphology of the morphospecies in this study, as exemplified in Figure 6 View Figure 6 . This is mostly attributable to our strict concept of T. trilobus regarding the dominance of the final chamber; it must be larger than all of the preceding chambers combined (sensu Bolli 1957) (see Morphometrics and biometrics). Trilobatus trilobus intergrades with T. immaturus , so using the relative size of the last chamber as the primary delimiting character is arbitrary. However, it can be estimated without using scanning electron microscopy and is easily quantified using morphometrics, thus making it an excellent discerning feature. In the morphometric results, a T. trilobus final chamber dominance ratio ( FCDR) must be greater than or equal to 1, whereas in T. immaturus the FCDR value must be less than 1 ( Fig. 19 View Figure 17 ). The ratio compares chamber area (Lm 2) from a two-dimensional image (see Methods). In T. trilobus , the final chamber volume should also be larger than the rest of the test volume. As the final chambers always have a regular, globose morphology (i.e. no flattening or extension as in T. sacculifer ), specimens with ratios of more than 1 can also be used to equate to a larger chamber volume (Lm 3) ratio. Typically, the maximum diameter of the final chamber is also greater than the maximum diameter of the rest of the test. However, occasionally the first two chambers of the final whorl appear marginally wider than the last chamber (e.g. Fig. 6D View Figure 6 ), yet this is still considered a T. trilobus because of the dominant last chamber (i.e. an FCDR value of> 1).
Conversely, if the final chamber is not larger than all preceding chambers, then the specimen cannot be T. trilobus . Essentially, this means that T. trilobus cannot possess a kummerform final chamber (sensu Berger 1969; see also Olsson 1973). This consequently results in many specimens with kummerform final chambers being assigned to T. immaturus (e.g. Fig. 7A–D View Figure 7 ; see T. immaturus , Remarks).
Trilobatus trilobus has a sacculifer - type wall texture ( Fig. 6C View Figure 6 ), although this is commonly obscured by secondary, ‘gametogenic’ calcite. In particular, the initial chambers of the final whorl are more heavily calcified and the sacculifer - type wall texture is less evident (e.g. Fig. 6C View Figure 6 ). Secondary calcification also affects the preceding whorl on the spiral side, and the small chambers are often indistinct. If the preceding whorl cannot be clearly observed to identify the spiral side, it is distinguished by the position of the largest aperture. The umbilical side possesses an extraumbilical-umbilical aperture, whereas the spiral side has a central supplementary aperture placed at the base of the final chamber at the sutures of the preceding and third-preceding chambers.
Berger, W. H. 1969. Kummerform foraminifera as clues to oceanic environments (abstract). Bulletin of the American Association of Petroleum Geologists, 53, 706.
Bolli, H. M. 1957. Planktonic foraminifera from the Oligocene - Miocene Cipero and Lengua Formations of Trinidad, B. W. I. Pp. 97 - 123 in A. R. Loeblich Jr, H. Tappan, J. P. Beckmann, H. M. Bolli, E. Montanaro Gallitelli & J. C. Troelsen (eds) Studies in Foraminifera. United States National Museum Bulletin, Smithsonian Institution, Washington DC, 213.
Bolli, H. M. & Saunders, J. B. 1985. Oligocene to Holocene low latitude planktic foraminifera. Pp. 155 - 262 in H. M. Bolli, J. B. Saunders & K. Perch-Nielsen (eds) Plankton stratigraphy. Cambridge University Press, Cambridge.
Brady, H. B. 1877. Supplementary note on the Foraminifera of the Chalk (?) of the New Britain Group. Geological Magazine, New Series, Decade 2, 4, 534 - 536.
Cushman, J. A. 1927. An outline of the re-classification of the foraminifera. Contributions from the Cushman Laboratory for Foraminiferal Research, 3, 1 - 105.
d' Orbigny, A. 1846. Foraminif`eres fossils du Bassin tertiaire de Vienne (Autriche). Gide et Comp, Paris, 312 pp.
Kennett, J. P. & Srinivasan, M. S. 1983. Neogene planktonic Foraminifera, a phylogenetic atlas. Hutchinson Ross, Stroudsburg, Pennsylvania, 265 pp.
LeRoy, L. W. 1939. Some small foraminifers, ostracoda and otoliths from the Neogene (' Miocene') of the Rokan-Tapanoeli area, Central Sumatra. Natuurkundig Tijdschrift voor Nederlandsch-Indi ¨ e, 99, 215 - 296.
Olsson, R. K. 1973. What is a kummerform planktonic foraminifer? Journal of Paleontology, 47, 327 - 329.
Reuss, A. E. 1850. Neue Foraminiferen aus den Schichten des osterreichischen Terti ¨ arbeckens. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe, 1, 365 - 390.
Reuss, A. E. 1867. Die fossile Fauna der Steinsalzablagerungen von Wieliczka in Galizien. Sitzungsberichte der kaiserlichen Akademie der Wissenschaften in Wien, mathematisch-naturwissenschaftliche Classe, 1. Abtheilung, 55, 17 - 82.
Spezzaferri, S., Kucera, M., Pearson, P. N., Wade, B. S., Rappo, S., Poole, C. R., Morard, R. & Stalder, C. 2015. Fossil and genetic evidence for the polyphyletic nature of the planktonic Foraminifera ' Globigerinoides ', and description of the new genus Trilobatus. PLoS ONE, 10, e 0128108.
Figure 6. Typical Trilobatus trilobus and intergradation with Trilobatus immaturus. A–O, Trilobatus trilobus (Reuss, 1850); P, Trilobatus immaturus (LeRoy, 1939). A–K, GLOW-3, south-west Indian Ocean (A, D, E, G, I, spiral view, in D note no spine holes are visible due to gametogenic calcite; B, F, H, J, K, umbilical view, in F note thick gametogenic calcite obscuring sacculifer-type wall texture on penultimate chamber; C, detail of coarse sacculifer-type wall texture and abundant spine holes at intersections of interpore ridges); L, M, ODP Site 926, Ceara Rise, western tropical Atlantic, 11H/04/50–52 cm (umbilical view); N, O, ODP Site 871, Limalok Guyot, Marshall Islands, equatorial Pacific 3H/03/60–62 cm (N, umbilical view; O, detail of gametogenic calcite, no spine holes visible). P, ODP Site 871, Limalok Guyot, Marshall Islands, equatorial Pacific 3H/03/60–62 cm (umbilical view; note final chamber not dominant over previous chambers, compare with A–O). Scale bars = 100 Lm, except for close-up images C and O, where scale bars = 20 Lm.
Figure 7. Typical Trilobatus immaturus and intergradation with Trilobatus quadrilobatus. A–K, Trilobatus immaturus (LeRoy, 1939); L–N, Trilobatus quadrilobatus (d’Orbigny, 1846). A–C, I–K, ODP Site 926, Ceara Rise, western tropical Atlantic, 11H/04/ 50–52 cm (A, B, umbilical view; C, detail wall texture and infilled pores; I, spiral view; J, detail of final chamber wall texture; compare with K, detail of penultimate chamber where primary wall texture is obscured by thick gametogenic calcite); D–H, GLOW- 3, south-west Indian Ocean (D, detail of wall texture and imperforate lip on first supplementary aperture; E, H, umbilical view; F, G, spiral view). L–N, GLOW-3, south-west Indian Ocean (L, spiral view; M, umbilical view; N, detail of wall texture including clear spine holes). Scale bars = 100Lm, except for close-up images C, D, J, K, N, where scale bars = 20 Lm.
Figure 17. Trilobatus sacculifer plexus, showing type specimens (A–D), morphological intergradation sequence and morphospecies exemplars (E–H). A, E, Trilobatus trilobus (Reuss, 1850); B, F, Trilobatus immaturus (LeRoy, 1939); C, G, Trilobatus quadrilobatus (d’Orbigny, 1846); D, H, Trilobatus sacculifer (Brady, 1877). A, salt mine Wieliczka, near Krakow, Poland (umbilical view; neotype image reproduced from Rogl 2012, pl. 1, fig. 1). B, Telisa Shales, Tapoeng Kiri area, Rokan-Tapanoeli, Central Sumatra, Indonesia (umbilical view; holotype image reproduced from LeRoy 1939, pl. 3, fig. 19). C, Nussdorf (= Nubdorf), Rara, Vienna Basin, Austria (umbilical view; lectotype image reproduced from Papp & Schmid 1985, pl. 3, fig. 19). D, New Ireland, Papua New Guinea (umbilical view; lectotype of Banner & Blow 1960, image reproduced from Williams et al. 2006, pl. 1, fig. 1). E, GLOW-3, south-west Indian Ocean (umbilical view). F, ODP Site 871, Limalok Guyot, Marshall Islands, equatorial Pacific 3H/ 03/60–62cm (umbilical view). G, ODP Site 871, Limalok Guyot, Marshall Islands, equatorial Pacific 3H/03/60–62 cm (umbilical view). H, ODP Site 1115, Woodlark Basin, western Pacific; 11H/04/25–27 cm (umbilical view). Scale bars = 100 Lm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |