Cryptorhynchinae
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3718.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:39B2DCEE-52B6-435E-9A3E-3CD5F55EFD36 |
|
DOI |
https://doi.org/10.5281/zenodo.6151983 |
|
persistent identifier |
https://treatment.plazi.org/id/692487D1-FF98-FFAE-FF4D-FAABC174E37C |
|
treatment provided by |
Plazi |
|
scientific name |
Cryptorhynchinae |
| status |
|
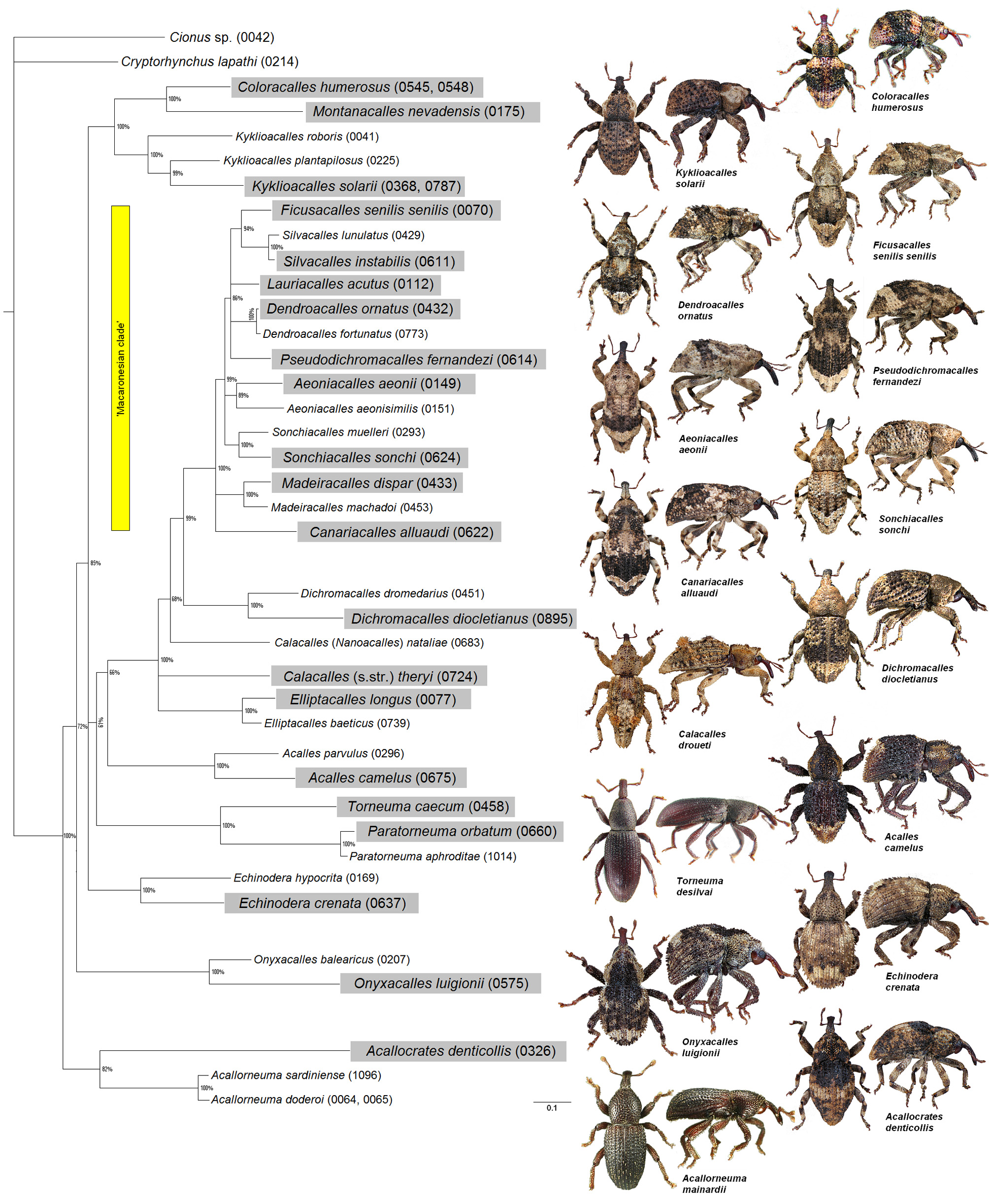
3.1. Cryptorhynchinae tree
( Fig. 1 View FIGURE 1 )
Molecular analysis is based on 39 species. Concatenated sequences of COI, 16S and 28S (of D6-D7 domain) gene fragments were generated. Fourty two specimens were used in total, because for three species we needed two specimens each to provide all three gene sequences. Two flying outgroup species are included: Cionus sp. ( Curculionidae : Curculioninae ) and Cryptorhynchus lapathi ( Curculionidae : Cryptorhynchinae ). Collecting and vouchering information as well as GenBank accession numbers are given in Table 2 View TABLE 2 . Voucher specimens and extracted genomic DNA are deposited at the Biobank of the Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK). The laboratory routine followed ASTRIN & STÜBEN (2008). PCR primers were taken from ASTRIN & STÜBEN (2008), COI primer set is based on the FOLMER et al. (1994) region; 16S primer set is based on CRANDALL & FITZPATRICK (1996), 28S primer set was developed in ASTRIN & STÜBEN (2008). For detailed primer information see Table 3 View TABLE 3 . As DNA barcoding region we define here the COI sequence which is, relative to the mouse mitochondrial genome, the 648 nucleotide (nt) region that starts at position 58 and stops at position 705 of cytochrome c oxidase subunit 1. This sequence area is commonly used for species identification in animal barcoding initiatives.
DNA sequence alignments for COI, 16S and 28S genes were performed with Geneious 5.5.6 Pro (DRUMMOND et al. 2012) using Muscle plugin with default parameters. Primer sequences were trimmed and single nucleotide polymorphisms and gaps of 16S and 28S alignments were manually shifted to minimize differences between sequences, especially to prevent gaps at the begining or end of a sequence. Missing data were filled up with “n” positions (whole gene or missing nucleotides in the beginning or end of a sequence). 16S sequence data were not available for one species ( Acallorneuma sardiniense, 1096 ). 28S sequence data were not available for two species ( Acallorneuma sardiniense, 1096 ; Paratorneuma aphroditae, 1014 ).
Poorly aligned positions and highly divergent regions (based on insertions or deletions) of 16S and 28S sequences were determined by Gblocks (CASTRESANA 2000; TALAVERA & CASTRESANA 2007) with three options activated for less stringent selection compared to basic settings: allowing smaller final blocks, allowing gap positions within the final blocks and allowing less strict flanking positions. The ambiguous positions were not provided to subsequent jModeltest analysis and also excluded in Bayesian analysis. This served the purpose of improving positional homology over the whole alignment, so that it becomes more suitable for phylogenetic analysis (WÄGELE 2005).
Alignment length was 658 nucleotides (nt) for COI, 524 nt for 16S (excluding ambiguous data), and 365 nt for 28S (excluding ambiguous data). The best fitting nucleotide substitution model to use in Bayesian analysis was determined for every single gene alignment using jModelTest ver. 0.1.1 (POSADA 2008) implementing the Bayesian Information Criterion (BIC; SCHWARZ 1978): for COI and 16S we identified the HKY+I+G (HASEGAWA et al. 1985), a submodel of the GTR+I+G, for 28S GTR+G (LANAVE et al. 1984); +G includes gamma distributed rates across sites, +I includes a proportion of invariable sites in the calculation.
Afterwards a concatenated sequence block was built from COI, 16S and 28S alignments. Poorly aligned positions of 16S and 28S were were excluded in the phylogenetic analysis, but were kept in the concatenated data block to ensure the reproducibility of the calculation based on the sequences of the corresponding Genbank accession numbers. The 16S data comprised eleven poorly aligned positions or regions (699–706, 808, 893–898, 909–910, 918–929, 944–945, 990–992, 1027–1033, 1075, 1132, 1160), the 28S data comprised seven (1250–1259, 1269–1272, 1352, 1499–1559, 1568–1571, 1578–1599, 1609–1651). Out of 1726 nucleotide positions 1537 were used for phylogenetic analysis.
We ran MrBayes ver. 3.1.2 (RONQUIST & HUELSENBECK 2003) in two independent replicates, each with 1 cold chain and 3 chains of different temperature (standard setting). For the COI sequence block, the genetic code for metazoan mitochondrial DNA was used for Bayesian analysis. All gene partitions were unlinked in shape, revmat, statefreq and pinvar. The calculation was performed for 40 million generations (average standard deviation of split frequencies: 0.0016), sampling 40.000 trees. Negative log-likelihood score stabilisation was determined in a separate visualisation in Microsoft Excel 2003. Accordingly, we retained 39.990 trees after burn in (10.000 generations were discarded), from which a 50%-majority rule consensus tree with posterior probabilities was built ( Fig. 1 View FIGURE 1 ). FigTree 1.3.1 (RAMBAUT et al. 2009) was used for graphical display of the tree.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.