Amphisbaena carli, Pinna, Pedro H., Mendonça, André F., Bocchiglieri, Adriana & Fernandes, Daniel S., 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.197346 |
|
DOI |
https://doi.org/10.5281/zenodo.6208179 |
|
persistent identifier |
https://treatment.plazi.org/id/762287ED-FFF7-C409-FF7A-F96EFA801F81 |
|
treatment provided by |
Plazi |
|
scientific name |
Amphisbaena carli |
| status |
sp. nov. |
Amphisbaena carli sp. nov.
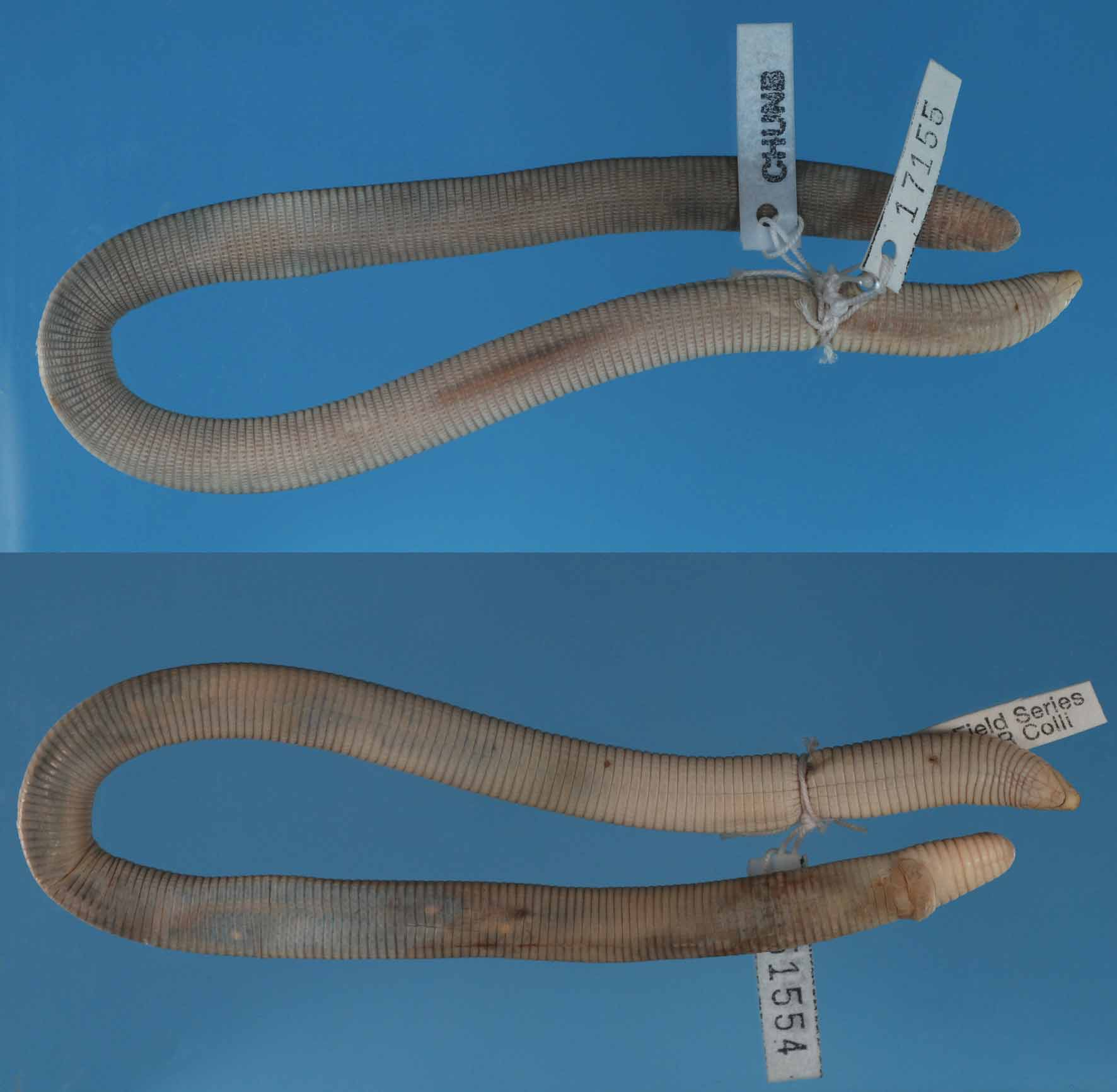
Holotype ( Fig. 2 View FIGURE 2 ). Adult male ( MNRJ 19256) collected in a recently deforested Pinus spp. plantation area at Jatobá farm, 14o01’S / 45o54’W, municipality of Jaborandi, southwestern Bahia, at 10:30 on 11 April 2009 by A. Bocchiglieri.
Paratypes ( Fig. 3 View FIGURE 3 ). Adult male ( MNRJ 19257) found road killed between a Cerrado fragment and a Pinus spp. plantation area at Jatobá farm, 14o03’S / 45o52’W, on 30 January 2008 by A. Bocchiglieri and A. F. Mendonça.
Adult, undetermined sex, ( CHUNB 51554) collected in the municipality of Cocos, southwestern Bahia, on 5–6 November 2006 by G. Colli.
Etymology. Amphisbaena carli is named after Carl Gans (in memoriam), a researcher who contributed immensely to the knowledge of amphisbaenians and whose works were fundamental to the production of this paper.
Diagnosis. Amphisbaena carli is diagnosable from all congeners by the following conjunction of characters: (1) small nasals separated from each other by the rostral; (2) two pre-cloacal pores separated from each other by the cloacal shield; (3) 221–242 body annuli; (4) 10–13 caudal annuli; (5) 21–23 dorsal and 21– 23 ventral segments to a midbody annulus; (6) tail without visible autotomic constriction.
Comparisons are based on literature data ( Gray 1865; Gans 1964; Gans 1971; Vanzolini 1971; Vanzolini 1991b; Vanzolini 1992; Strüssmann e De Carvalho 2001; Vanzolini 2002; Castro-Mello 2003; Hoogmoed et al. 2009; Mott & Vieites 2009; Ribeiro et al. 2009) and specimens examined (Appendix). In order to make comparisons clear, species were separated by groups reflecting the taxonomy before Mott and Vieites (2009).
Amphisbaena carli is distinguished from Mesobaena huebneri Mertens and M. rhachicephala Hoogmoed et al. by having a rounded rostrum, instead of a keel-shaped rostrum in both species of Mesobaena . It also differs from the species of Amphisbaena formally placed at the genus Anops — Amphisbaena bilabialata (Stimson) , A. kingii (Bell) and A. acrobeles (Ribeiro et al.) —by having a rounded snout against a vertically keeled rostrum in the later species. It differs from the species of Amphisbaena formally placed at the genus Aulura — Amphisbaena anomala (Barbour) —by having a rounded snout against a shovel-shaped rostrum in the later species. It differs from the species of Amphisbaena formally placed at the genus Leposternon — Amphisbaena infraorbitale (Berthold) , A. kisteumacheri (Porto et al.) , A. microcephalum (Wagler) , A. octostegum (Duméril) , A. polystegum (Duméril) , A. scutigerum Hemprich and A. wuchereri (Peters) — by having a rounded snout against a shovel-shaped rostrum in the later species. It differs from the species of Amphisbaena formally placed at the genus Cercolophia — Amphisbaena absaberi (Strüssmann & de Carvalho), A. bahiana Va nz o l in i, A. borellii Peracca , A. cuiabana (Strüssmann & de Carvalho), A. roberti Gans and A. steindachneri Strauch—by having no keeled tail, present in the later species.
Amphisbaena carli shares with the species of Amphisbaena formally placed at the genus Bronia — Amphisbaena bedai (Vanzolini) , A. brasiliana , A. kraoh (Vanzolini) and A. saxosa — the strongly curved rostrum. Besides, it also shares with A. brasiliana and A. saxosa the nasals separated from each other by the rostral. However, it can be easily distinguished from these four species by having only two pores (against six in A. kraoh and four in A. brasiliana , A. bedai and A. saxosa ) and 240–242 body annuli (against 213–229 in A. brasiliana , 253–272 in A. saxosa , 272–284 in A. bedai , and 281 in A. kraoh ). It differs from the two-pored species formally placed at the genus Amphisbaena — A. anaemariae Vanzolini, A. brevis Strüssmann & Mott , A. crisae Vanzolini , A. darwini Duméril & Bibron , A. dubia Müller , A. heterozonata Burmeister , A. hiata Montero & Céspedez , A. leeseri Gans , A. miringoera Vanzolini , A. mitchelli Procter , A. neglecta Dunn & Piatt , A. silvestrii Boulenger and A. trachura Cope—by having small nasals separated from each other by the rostral (against large nasals touching each other on the midline in the later species). It is further distinguishable from A. anaemariae , A. brevis , A. crisae , A. darwini , A. dubia , A. heterozonata , A. mitchelli , A. neglecta , A. silvestrii and A. trachura by having 240–242 body annuli (against less than 231 in the other species). Amphisbaena carli can also be distinguished from A. miringoera by having 12–13 caudal annuli (against 22–24) and from A. hiata and A. leeseri , by having no autotomic site (present in those species).
Description of the holotype ( Fig. 4 View FIGURE 4 ). Adult male, snout-vent length (SVL) 264 mm, tail length (TL) 15 mm. Head dorsally triangular and poorly distinct from the neck which is slightly narrower than the rest of the body; rostrum extremely rounded, projecting forward beyond the jaw (prognathous snout); rostral well visible in dorsal view, broader than long and in contact with nasals and frontals; anterior portion very rounded; small posterior projection toward the suture between frontals; laterally rostral forming a concave curve around the anterior portion of nasals, contacting first supralabial; nasals separated by the rostral, not meeting each other on the midline; nasals contacting first supralabial; frontals paired, touching each other on the midline, longer than broad, being the largest shields on the top of the head (2.5 mm long at ventral edge); frontals with postero-lateral projections above the oculars toward the parietals; laterally frontals in a narrow contact with the first and second supralabials; parietals paired, large, touching each other on the midline and with lateral projections towards the oculars; occipitals absent; after parietals follows the first body annulus with the dorsalmost segments nearly square, slightly larger than the rest of the body segments; four supralabials, second and third touching ocular; the first three diagonally oriented and larger than the fourth, which does not participate on the formation of the labial comissure but is herein considered as a supralabial for its position above the third infralabial; two temporals on each side of the head arranged in a single row; upper temporal slightly higher than lower temporal and visible from above; there is an azygous shield at the left upper temporal; lower temporal about twice higher than fourth supralabial to which it is in contact; lower temporal also contacts the third supralabial and a very small portion of the ocular; oculars nearly triangular; eyes visible dorsally and laterally, located at the postero-dorsal portion of the oculars, near the contact with parietals. In ventral view symphysial anvil-shaped, separating first pair of infralabials; postsymphysial wider than long (1.7 / 1.4 mm respectively) and posteriorly rounded, separating the second pair of infralabials; three infralabials, first small, second occupies most of the labial comissure, and third extends to some ventral segments of the first body annulus; four genials in a single row; lateral genials broader than long and median genials longer than broad; eight postgenials, the outermost pair broader than the six inner scales; 240 body annuli; 13 caudal annuli; tail relatively blunt and short, no evidence of autotomy site; four lateral incomplete annuli between the last body annulus and the first caudal one; lateral sulci well visible, more noticeable after the first third of the body; 21 dorsal and 21 ventral segments (counted at a midbody annulus); dorsal and ventral sulci absent; cloacal shield covered by six segments decreasing in size from the larger medial pair; two well visible pre-cloacal pores separated one from the other; each pore located at a single, small scale next to the two outermost pairs of segments of the cloacal shield. The pore-bearing scales (as well as the cloacal shield) are part of the first incomplete annulus. Dorsal ground color of the head and body creamish white; small dark dots visible at the anterior half of the medial dorsal segments, more noticeable at the posterior half of the body and also at the dorsum of the tail; venter creamish white, immaculate.
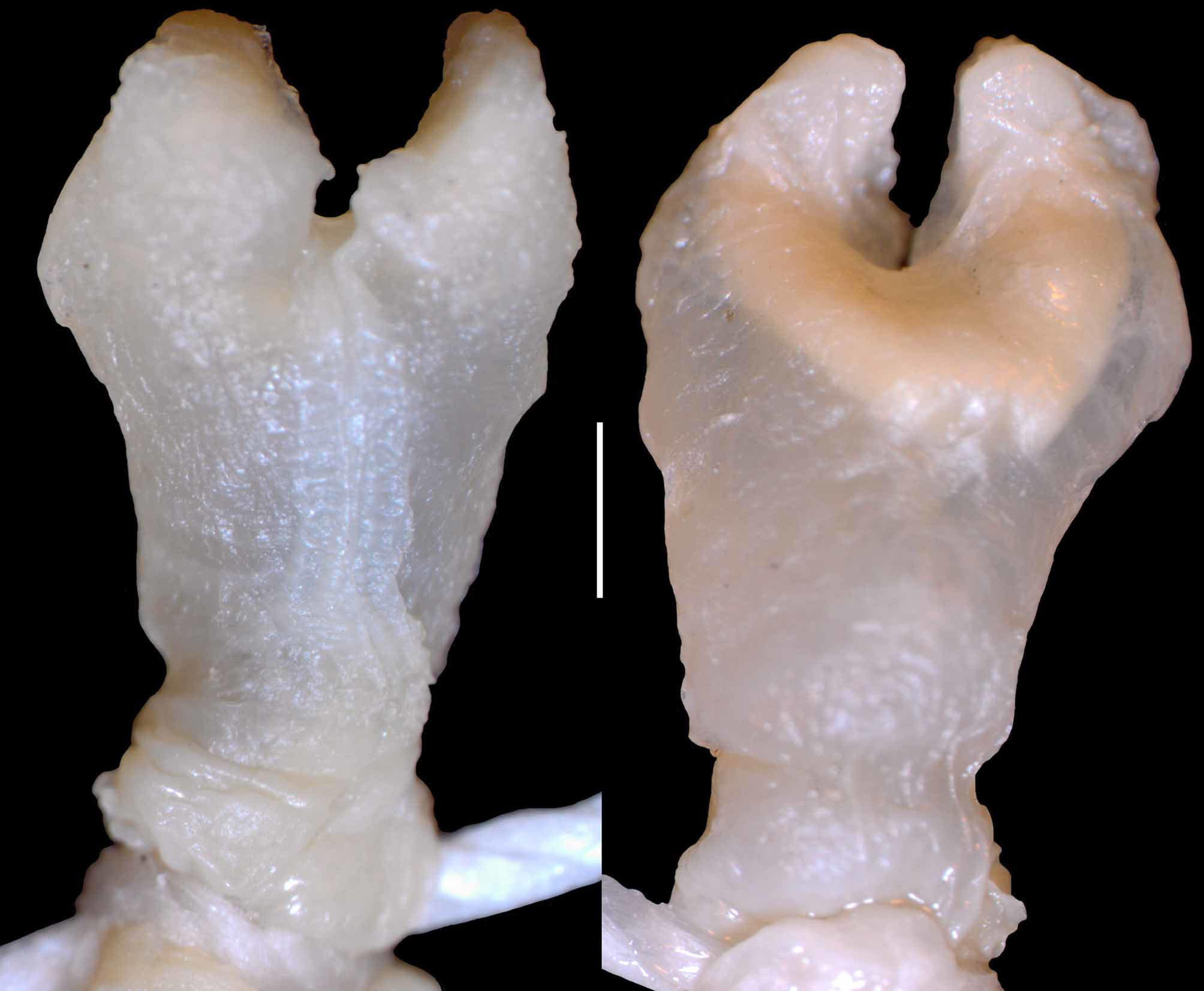
Description of the hemipenis ( Fig. 5 View FIGURE 5 ). Bilobed, with no capitulum or ornamentations but with a wrinkled surface. Lobes correspond to about one third of hemipenial body, with simple rounded tips slightly curved towards the asulcate side. Sulcus spermaticus with prominent lips bifurcates at the base of the lobes, each branch following to the tip of a lobe in a centrolineal direction. On the asulcate side a dense semi-circle of inner tissue is well visible by transparency at the branching point of the organ projecting inside both lobes.
Variation. The paratype from Jaborandi (MNRJ 19257; SVL = 171 mm and TL = 10 mm) has 242 body and 12 caudal annuli and 22 dorsal and ventral segments. The paratype from Cocos (CHUNB 51554; SVL = 227 mm and TL = 13 mm) has 221 body and 10 caudal annuli and 23 dorsal and ventral segments. Others characters and color pattern are similar to the holotype for both specimens.
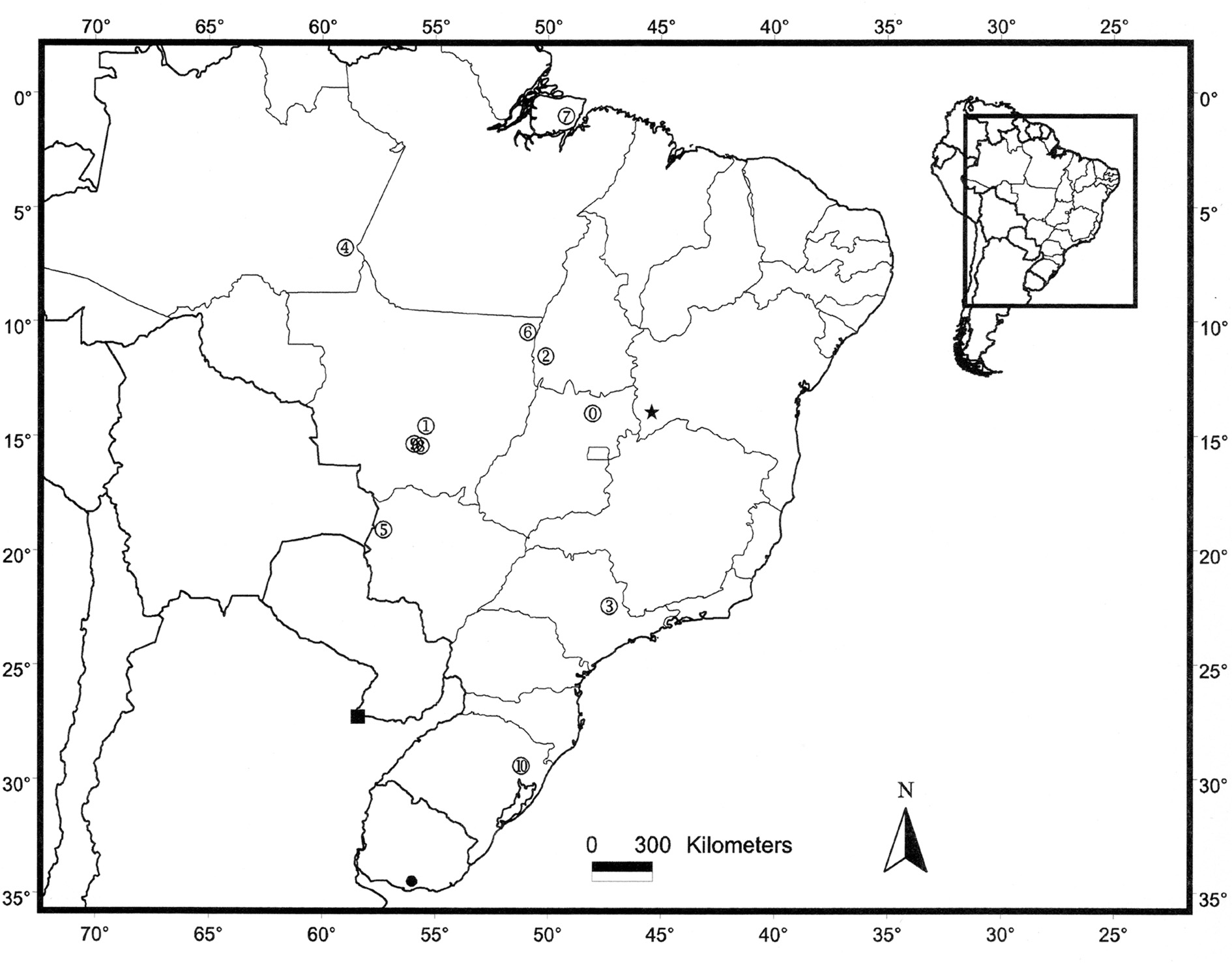
Distribution ( Fig. 6 View FIGURE 6 ). The new species is known from its type locality, Jatobá farm (13º53’S / 45º42’W), municipality of Jaborandi, Bahia state, near the frontier with the state of Goiás. The area corresponds to the Chapadão Ocidental of the São Francisco River at the region called “Gerais”. The farm is bounded by two rivers (Arrojado and Veredãozinho) that are part of the São Francisco basin and has a total area of 92,000 ha. In the decade of 1980, the farm started planting Pinus spp. and Eucalyptus spp., occupiyng 40,000 ha of the total area, the remaining area (52,000 ha) being kept as cerrado (like an open savannah) biological reserves ( Fenger & Sevensson 2004). It is interesting to point out that the holotype was found in a Pinus spp. planting area while the paratype from Jaborandi was found road-killed nearby a Cerrado fragment. Nevertheless, both habitats are endangered once they are being deforested since the beginning of 2008 to make room for soy plantation, which at the present time represents the main activity of the farm. Nowadays, the remaining area of cerrado (30,000 ha—22,000 ha less than in the 80´s) are confined to the rivers bounding the farm (gallery forest) and rocky environments, the latter representing an unfavorable habitat for amphisbaenids. Amphisbaena carli is also known from Cocos, state of Bahia, a neighboring municipality to Jaborandi, approximately 180 kilometers northeast from Jatobá farm. The specimen of this locality was found in a cerrado area with sandy soil, similar to the environment where specimens from Jatobá farm were collected.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.