Amynthas chilanensis Tsai and Tsai, 2007
|
publication ID |
https://doi.org/ 10.1080/00222930701201279 |
|
persistent identifier |
https://treatment.plazi.org/id/8B4B87BE-FF9A-D91C-9E69-1A5400FC7D8F |
|
treatment provided by |
Felipe |
|
scientific name |
Amynthas chilanensis Tsai and Tsai |
| status |
sp. nov. |
Amynthas chilanensis Tsai and Tsai , sp. nov.
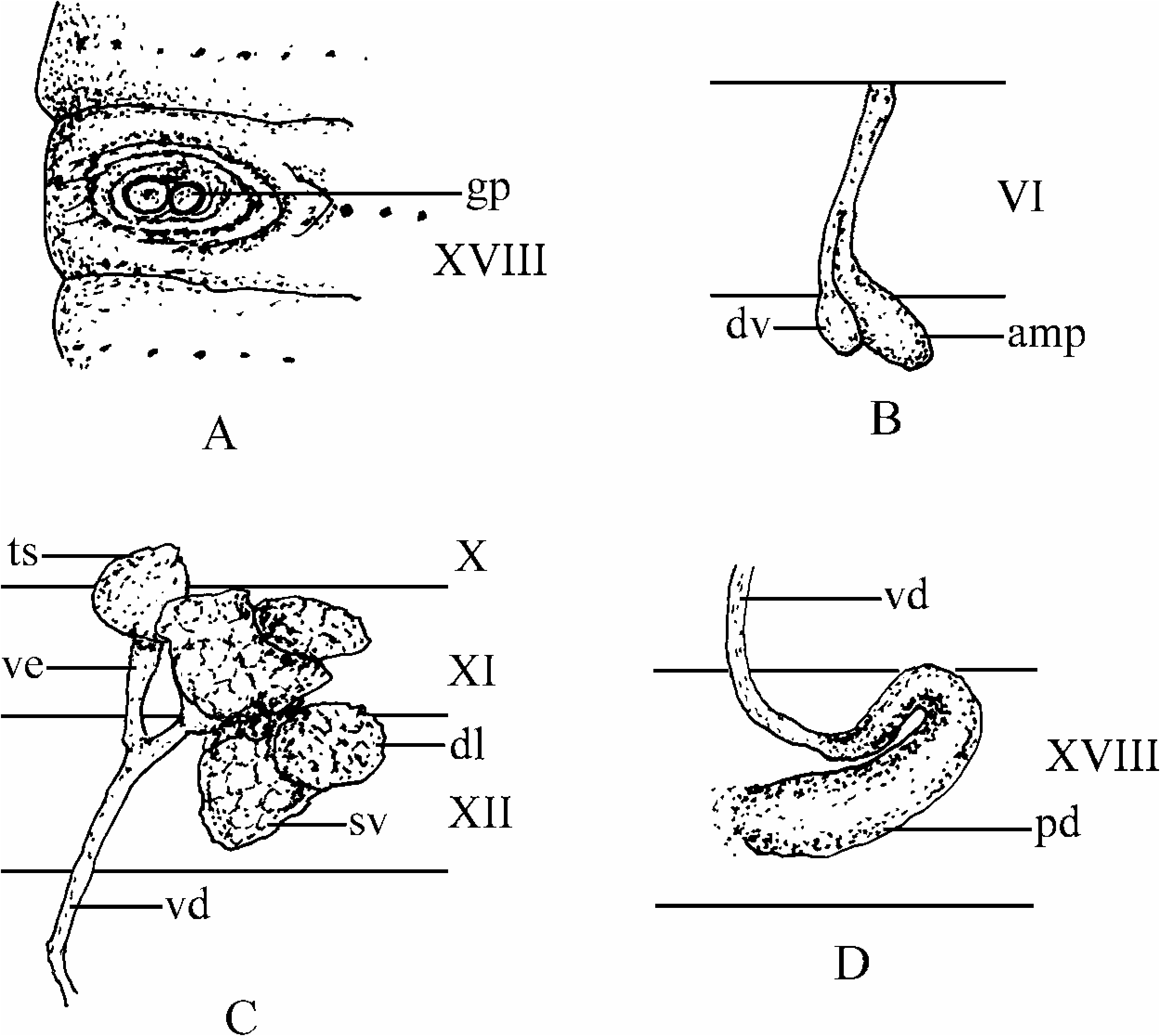
( Figure 2 View Figure 2 )
Type material
Holotype: a clitellate specimen (168 mm, dissected) collected 17 October 2002 along the No. 100 Forest Road (elevation 1325 m), Chilan, Ilan County by C. F. Tsai, S. C. Tsai, H. P. Shen, S. T. Chang, R. C. Jang, B. H. Lin, and C. Y. Chang (coll. no. 2002-25-Shen) . Paratypes: a clitellate specimen (133 mm, dissected) and an aclitellate specimen (93 mm) (same collection data as holotype).
Diagnosis
Medium earthworm; length (clitellates) 133–168 mm, segments numbering 88–116. Setae 32–35 in VII, 47–50 in XX, and 11–12 between male pores. Spermathecal pores absent. No genital papillae in the preclitellar region. Male pores 0.26–0.32 body circumferences ventrally apart, each on a small oval porophore with a round or oval-shaped genital papilla with a depressed centre on setal line immediately medial to porophore, and then surrounded by three to four skin folds. Spermathecae usually absent (athecate). Seminal vesicles small. Prostate glands absent. Prostatic duct U-shaped with an enlarged distal end. No accessory glands. Parthenogenetic.
External characters
Total length 133–168 mm for clitellates and 93 mm for aclitellate. Clitellum constricted, 3.38–4.19 mm in length and 4.71–4.84 mm in width. Prostomium epilobous. Segments numbering 88–116. Setae minute, 32–35 in VII, 47–50 in XX, and 11–12 between male pores in XVIII. First dorsal pore in 11/12.
Spermathecal pores absent. No genital papillae in the preclitellar region. Female pore single, medio-ventral in XIV. Male pores paired in XVIII, ventro-lateral, 0.26–0.32 body circumferences apart. Each pore on a small, oval, disc-like porophore, with a small round or slightly oval-shaped genital papilla with a depressed centre, that is equal in size to or slightly smaller than that of the male porophore, on setal line immediately medial to porophore, and then surrounded by three to four oval skin folds ( Figure 2A View Figure 2 ). Preserved specimens purplish brown on dorsum and light brown on ventrum. Clitellum dark chocolate in colour.
Internal characters
Septa 5/6–7/8 and 10/11–13/14 thickened, 8/9/10 missing. Nephridial tufts thick on anterior faces of 5/6/7 septa. Gizzard large in IX–X for clitellates but small in X for aclitellate. Oesophageal hearts enlarged in X–XIII. Intestine starting enlarged in XV. Intestinal caeca paired in XXVII, each simple with a wide base, extending anteriorly to XXIII or XXIV.
Spermathecae absent for 133-mm clitellate and 93-mm aclitellate, but a single vestigial spermatheca in right VI for 168-mm clitellate. The vestigial spermatheca small, with an oval ampulla and a long, straight stalk, and its diverticulum small, with a small oval seminal chamber and a short, straight stalk attached in the middle of the spermathecal stalk ( Figure 2B View Figure 2 ). Ovaries in XIII, paired, large with follicular surface.
Holandry: testis sacs large, two pairs in X and XI. Vas efferens short, thick (swollen), joining in XII or XIII on each side to form a large, straight vas deferens, connecting to prostatic duct in XVIII. Seminal vesicles two pairs in XI and XII, small (degenerated), follicular surface, pink in colour, each with a large dorsal lobe either reddish brown or whitish in colour ( Figure 2C View Figure 2 ). Prostate glands absent for all specimens, but prostatic ducts U-curved, distal end enlarged, silver coloured ( Figure 2D View Figure 2 ). No accessory glands detected.
Etymology
The name chilanensis is given to this species with reference to its type locality at Chilan , Ilan County in northeastern Taiwan .
Remarks
According to the classification of parthenogenetic morphs of earthworms ( Gates 1956, 1972), two specimens of A. chilanensis are I 5 morph, an intermediate between A morph (athecate) and AR morph (athecate and anarsenosomphic), and one specimen is late I 4 morph between H morph and AR morph. The former are athecate but the latter has a vestigial spermatheca in VI. Although all specimens are aprostatic, they have prostatic ducts, so that they are not considered as R morph.
Amynthas chilanensis is closely related to A. sheni from Hong Kong ( Chen 1935). However, eight specimens of A. sheni are typical athecate A morph with well-developed male terminalia, prostate glands, preclitellar genital papillae, and large accessory glands (Table II). According to the phylogenetic species concept ( Cracraft 1989; Kullander 1999), species delimitation for parthenogenetic animals by the approach of Gates (1972) and views of Frost and Wright (1988) and Frost and Hillis (1990), A. sheni in Hong Kong and A. chilanensis in Taiwan are considered as two phylogenetic species. They are two clusters that have distinct diagnosable characters without intermediates, and each cluster has its own independent evolutionary lineage and direction (morphologically and biogeographically). See the species delimitation for parthenogenetic earthworms in discussion section.
Gates (1972, p 217) indicates that athecate individuals of sheni ( Chen, 1935) are likely to be referable to diffringens (5 corticis View in CoL ) or to robustus than to hawayanus (5 gracilis ) or morrisi. However, A, R, and AR morphs if seen were never recognized as belonging to robustus. This ‘‘likely but not so’’ statement was interpreted by Blakemore (2003, p 16) to mean that Gates (1972) suggested sheni may be an athecate morph of either robustus or cortici s, mostly likely the latter, so that he assigned sheni as a synonym with question mark to both corticis View in CoL and robustus. Later Blakemore et al. (2006) further stated that this questionable synonym of sheni to robustus was proposed by Gates (1972).
For linking sheni to its possible H morph, when Chen (1935, p 41) erected the species he indicated that there is no intermediate to associate the athecate sheni with some typical species, and its general characters are very much like Pheretima pingi Stephenson, 1925 of Checking, China, but both are distinguishable specifically. The lack of intermediates might also be one of the reasons that Gates (1972, p 217) speculated but did not assign sheni as a synonym or a possible synonym of either robustus or corticis .
Amynthas sheni is distinguishable specifically from robustus simply by the character of genital papillae at male pores. Amynthas sheni has a single genital papilla (never paired) medial to each porophore, the consistent specific character ( Chen 1935, p 39) like that of chilanensis ( Figure 2A View Figure 2 ), whereas robustus usually has two genital papillae at each male pore, one presetal and one postsetal, others paired (apparently never median and unpaired) ( Gates 1972, p 216). Also, in this study A. chilanensis of Taiwan, closely related to A. sheni of Hong Kong, has a rudimentary spermatheca in VI. This may imply that the athecate morph of sheni , like chilanensis , is derived from an ancestral H morph which had spermathecae in VI, unlike the quadrithecate robustus that has spermathecae only in VIII and IX. Apparently, sheni is not referable to robustus, as once suspected by Gates (1972) and nearly accepted by Blakemore (2003) and Blakemore et al. (2006).
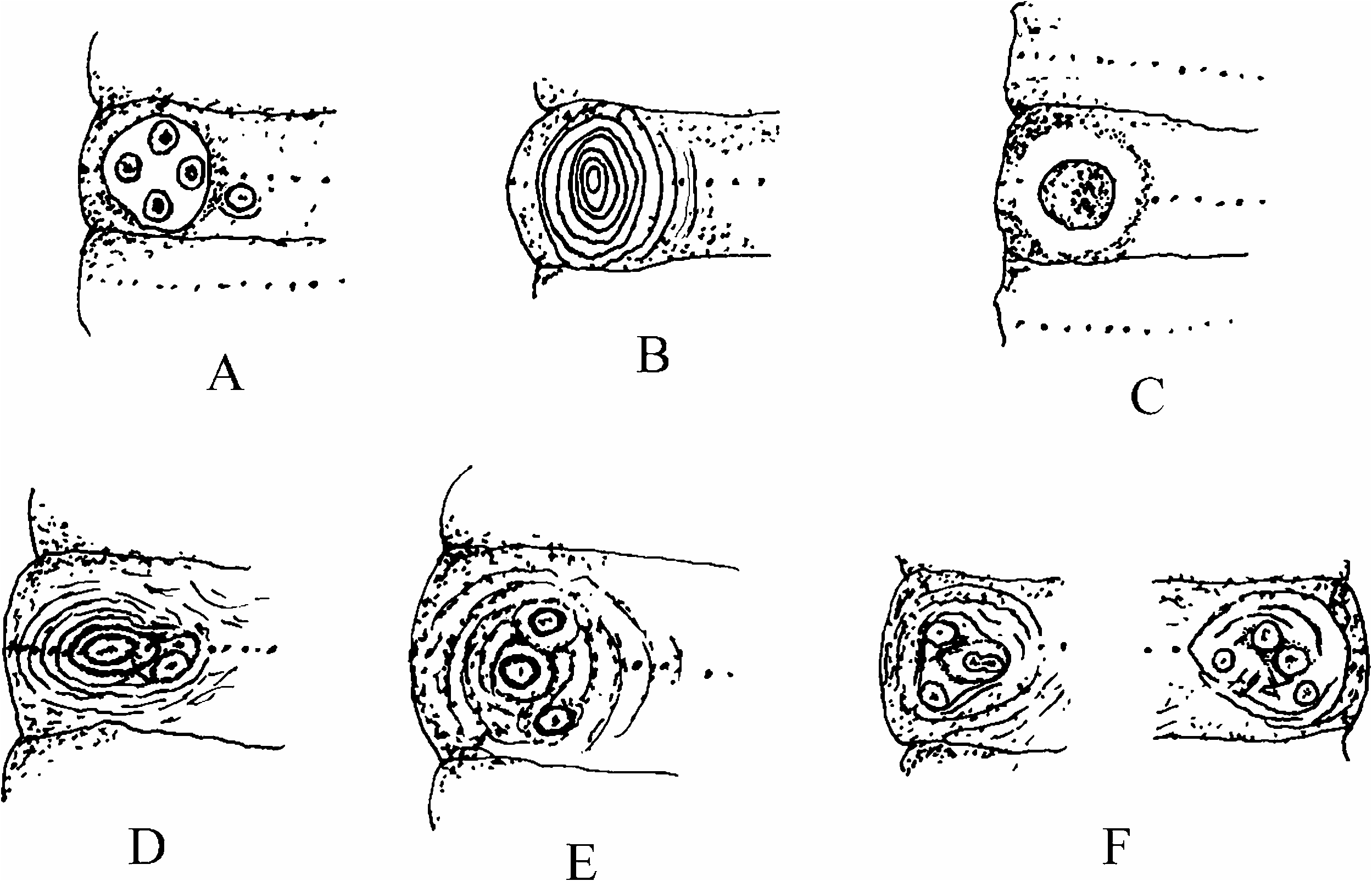
Generally, sheni fairly resembles octothecate corticis View in CoL as Gates (1972) indicated. The latter has spermathecae in VI. However, sheni is distinguishable from corticis View in CoL in that the former has a consistent genital papilla immediately medial to each male porophore as mentioned previously. For the latter the genital papillae at the male pore region are highly variable in number and location: absent to one or more in number ( Gates 1972, p 178), and one to two or three in number, in various arrangement for specimens from China ( Figure 5F View Figure 5 ) ( Chen 1933, p 235, Figure 16). In Taiwan corticis View in CoL has often one or two genital papillae in different arrangement ( Figure 5D, E View Figure 5 ) and specimens with three papillae are also observed; a single medial papilla is common ( Tsai 1964), but it is often postsetal on the first skin fold separated from the male porophore ( Figure 5D View Figure 5 ). Also, sheni is endemic to Hong Kong, and the closely related chilanensis is endemic to Taiwan, while corticis View in CoL is peregrine with worldwide distribution. According to the differences in structure of male pore region, habitat, and migratory behaviour, sheni shows no specific affinity with corticis View in CoL .
Actually, parthenogenetic morphs of sheni and chilanensis more resemble Amynthas taipeiensis ( Tsai, 1964) of Taiwan and of China (5 Pheretima heterogens Chen and Hsu, 1975 ) than robustus and corticis . The male pore structure with its genital papilla located immediately (contacted) medial to the male porophore of sheni and chilanensis ( Figure 2A View Figure 2 ) is almost identical to that of taipeiensis ( Tsai 1964, p 12, Figure 2 View Figure 2 ) of Taiwan and China ( Chen et al. 1975, p 90, Figure 2 View Figure 2 ). However, the latter is sexthecate and has spermathecal pores in 6/7–8/9. Also, taipeiensis is distinguishable specifically from sheni in that the former has higher setal numbers (46–52 in VIII and 61–63 in XX) than those of the latter (36–42 in VIII and 45–56 in XXV), and also the former has a long and coiled prostatic duct, whereas the latter has a U-shaped duct.
Based on the differences in male pore region and its associated genital papillae and general somatic characters mentioned above, neither robustus, corticis nor taipeiensis is likely to be the H morph of sheni and chilanensis . Furthermore, there are no intermediates in characters (numbers of spermathecae) that can link sheni (A morph) to either one of the latter three species to verify their specific affiliation as required ( Gates 1972). Without intermediates to substantiate the specific affiliation, to consider sheni as a possible synonym (with question mark) of either robustus or corticis ( Blakemore 2003; Blakemore et al. 2006), or even taipeiensis is simply a speculation. When the phylogenetic species concept ( Cracraft 1989; Kullander 1999) is applied to species delimitation for both hermaphroditic amphimictic morphs and parthenogenetic morphs, speculation on the ancestral H morph of sheni and chilanensis becomes meaningless.
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Amynthas chilanensis Tsai and Tsai
| Tsai, Chu-Fa, Shen, Huei-Ping, Tsai, Su-Chen & Lee, Hsun-Huang 2007 |
Amynthas chilanensis
| Tsai & Shen & Tsai & Lee 2007 |
A. chilanensis
| Tsai & Shen & Tsai & Lee 2007 |
chilanensis
| Tsai & Shen & Tsai & Lee 2007 |
A. chilanensis
| Tsai & Shen & Tsai & Lee 2007 |
chilanensis
| Tsai & Shen & Tsai & Lee 2007 |
chilanensis
| Tsai & Shen & Tsai & Lee 2007 |
corticis
| Kinberg 1867 |
corticis
| Kinberg 1867 |
corticis
| Kinberg 1867 |
corticis
| Kinberg 1867 |
corticis
| Kinberg 1867 |
corticis
| Kinberg 1867 |
corticis
| Kinberg 1867 |