Amynthas monsoonus, James & Shih & Chang, 2005
|
publication ID |
https://doi.org/ 10.1080/00222930400001434 |
|
persistent identifier |
https://treatment.plazi.org/id/03E61D4E-D63B-FF8D-57C4-CBCFF957FEFB |
|
treatment provided by |
Felipe |
|
scientific name |
Amynthas monsoonus |
| status |
sp. nov. |
Amynthas monsoonus sp. nov.
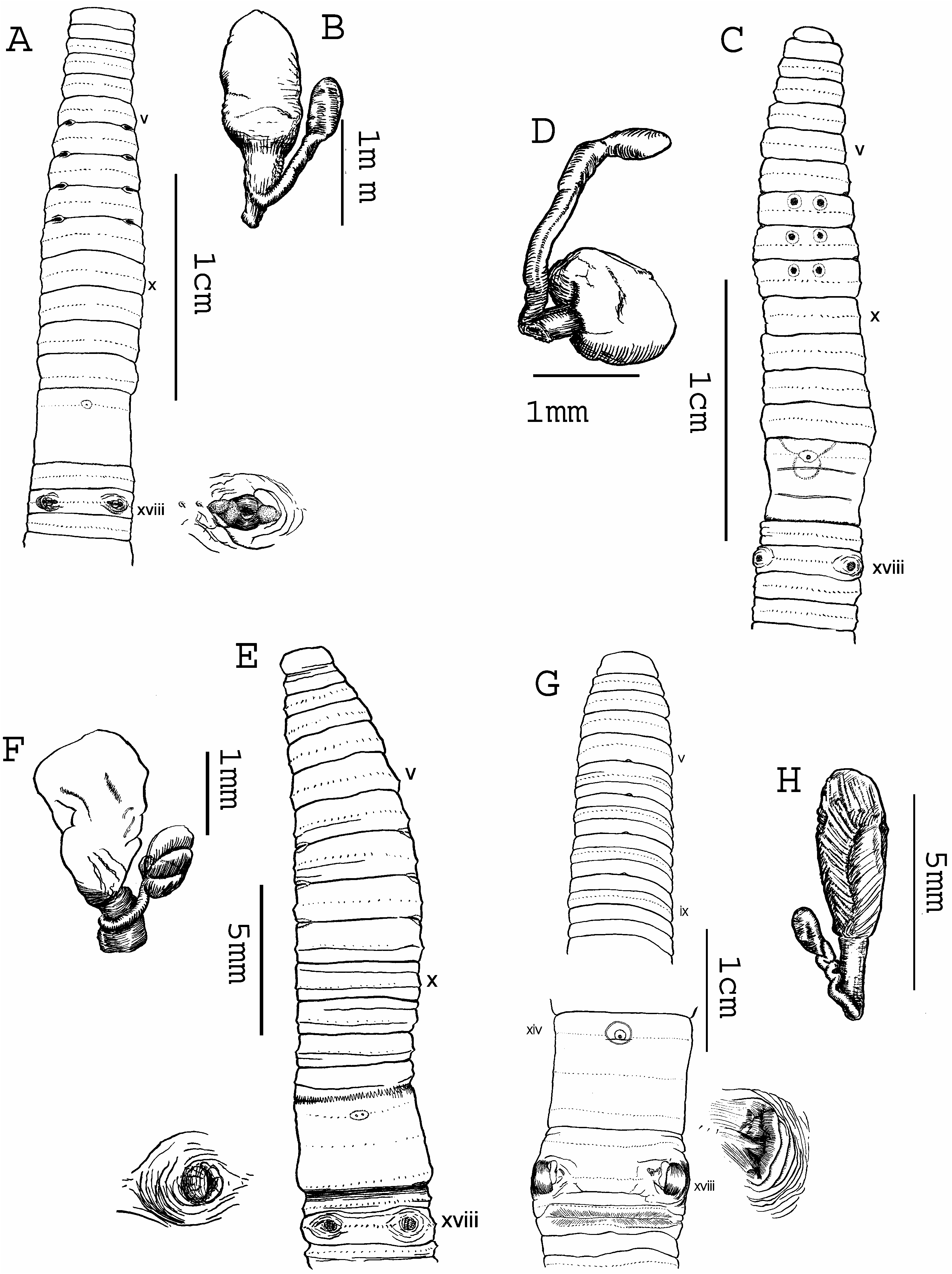
( Figure 2C, D View Figure 2 )
Holotype: one adult specimen collected 13 July 1998 at Nanrenshan , Kending, Pingtung County, Taiwan, 22 ° 059030N, 120 ° 509070E; 150 m by Chung-Chi Huang, NMNS 4054- 005 View Materials .
Etymology
The species is named for the tropical monsoon forest in Nanrenshan which is unusual for such a northern latitude.
Description
Dimensions 102 mm by 3.6 mm at segment x, 4.0 mm at xxx, 3.8 mm at clitellum; body cylindrical throughout, segments 83. Setae regularly distributed around segmental equators, numbering 38 at vii, 42 at xxv; size, interval regular; setal formula AA:AB:YZ:ZZ51:1:1:2 at xxv. Female pore single in xiv. Prostomium epilobic, with tongue open. Brown anterior dorsal pigmentation with unpigmented setal zones, pigment diminishing posteriorly but present to end. First dorsal pore 12/13. Clitellum annular xiv– xvi; setae invisible externally.
Male pores lacking associated genital markings, 16 setae between male pores. Spermathecal pores lateral in 6/7/8/9. Genital markings paired, presetal vii–ix between third, fourth setal lines ( Figure 2C View Figure 2 ).
Septa 6/7/8 thickly muscular, 8/9, 9/10 absent, 10/11–13/14 thinly muscular; gizzard viii– x. Intestinal origin xvi; lymph glands present from xxvi; typhlosole simple fold 0.5 lumen diameter from xxvii. Intestinal caeca simple, originating in xxvii, extending anteriorly to xxiv, small incisions on ventral margin. Oesophageal hearts two pairs in xii–xiii; hearts x, xi lacking; commissural vessels vii, ix lateral, viii to gizzard; extra-oesophageal vessels to ventral oesophageal wall in x.
Male sexual system holandric, testes, funnels in paired ventral sacs in x, xi. Seminal vesicles large in xi, xii, with dorsal lobe. Prostates small xviii, two main lobes, ducts thick, muscular, vasa deferentia join duct at duct–glandular portion junction; vasa deferentia nonmuscular.
Ovaries in xiii. Paired spermathecae in vii–ix; ampulla warty spherical, duct very short, diverticulum small ovate, stalk muscular, straight ( Figure 2D View Figure 2 ); no nephridia on spermathecal ducts; paired sessile genital marking glands vii–ix.
Remarks
In Sims and Easton (1972) this worm keys to the sieboldi group. Easton (1981) transferred A. sieboldi (Horst, 1883) to Metaphire , so the species group name may no longer be appropriate. On the other hand, the male pores of the type of M. sieboldi (National Natuurhistorisch Museum, Leiden, Netherlands, cat. no. 1825) are subapical on blunt cones surrounded by elevated circular lips leaving wide openings. Through this opening the cone is clearly visible. The circular trough surrounding the cone is wholly confined to the body wall, which shows no trace of bulging into the coelom. This leaves one in grave doubt about the validity of assigning A. sieboldi to Metaphire . Gates (1975, p. 7) wrote, ‘‘Presence or absence of copulatory chambers is too vague. The really important character is whether the male pores are superficial or invaginate. In the latter case, whether in slight transverse slits or much deeper spaces still confined to the parietes or whether thick-walled copulatory chambers deeply penetrating into coelomic cavity (cf Gates 1972, p. 150)’’. In Easton (1981) no details are given in support of the transfer, and he further commented on M. riukiuensis that it was uncertain if the male pores were in seminal grooves or copulatory pouches, so it was unclear if it should be placed in Amynthas or Metaphire . If the definition of ‘‘copulatory pouch’’ is so vague, then a critical review of the character and assignments of species to genera based on the character are clearly needed. We support following the suggestion of Gates (1975) to better characterize the status of various types of non-superficial male pores. For now we support restricting Metaphire to those species distinguishable from Pheretima only by the absence of nephridia from the spermathecal ducts ( Sims and Easton 1972). This would require the presence of well-developed copulatory pouches protruding into the coelom (as in Pheretima ), but leaves unclear what to do with species whose copulatory pouches are entirely intramural and could thus be distinguished from Pheretima .
Pending the outcome of these issues, the species group could be renamed the aelianus group after A. aelianus (Rosa, 1892) , that being the first in the species group list in Sims and Easton (1972). This group should also include six recently described species from Taiwan with spermathecal pores in 6/7/8/9: A. binoculatus Tsai, Shen and Tsai, 1999 , A. fenestrus Shen, Tsai and Tsai, 2003 , A. sexpectatus Tsai, Shen and Tsai, 1999 , A. tayalis Tsai, Shen and Tsai, 1999 , A. tenuis Shen, Tsai and Tsai, 2003 , and A. tungpuensis Tsai, Shen and Tsai, 1999 . Amynthas monsoonus differs from them all in lacking the anterior two pairs of hearts, like A. nanrenensis . It also has a different genital marking pattern from its Taiwanese sexthecal congeners. The missing hearts suggest that it is more closely related to A. nanrenensis than to the aelianus group members. No one has tested the hypothesis that the spermathecal battery is evolutionarily more conservative than details of the circulatory system, and there is evidence to the contrary. The locations of hearts are widely conserved among Amynthas , across great variation in other characters, particularly the numbers and locations of spermathecae. Amynthas monsoonus and the previous two species are quite unusual in having lost the hearts of x, or x and xi. These three are very similar with respect to other somatic characters and spermathecal morphology. Therefore it seems possible that their similarity is due to common ancestry, and that some species groups defined by spermathecal batteries could be polyphyletic or paraphyletic (addition or deletion of a pair would remove a taxon from its clade, rendering the latter paraphyletic). Advocates of sexual characters as indicators of phylogenetic relationships include most of the classical authors (prominent among them Michaelsen and Stephenson). Promotion of somatic characters for this purpose is one of the central themes of Gates’ work ( Gates 1972), but the question will not be decided without recourse to a third and independent set of characters. Nucleic acid sequence data are an obvious choice.
A suggestion that A. monsoonus is very similar to A. carnosus ( Goto and Hatai, 1899) discounts the more anterior location of the three or four pairs of spermathecae in the latter, as well as its possession of genital markings in segments xviii and xix, greater numbers of setae per segment, lack of genital marking glands, and the very different spermathecal morphology. Blakemore’s (2003) diagnosis of A. carnosus and subsequent remarks all place its first pair of spermathecal pores in 5/6.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |