Cebrennus rechenbergi, Jäger, Peter, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3790.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:BDA1931C-FEDB-4142-8A63-2765593621A9 |
|
DOI |
https://doi.org/10.5281/zenodo.6124571 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA87EF-FFB7-3964-FF69-FA7A83611AFB |
|
treatment provided by |
Plazi |
|
scientific name |
Cebrennus rechenbergi |
| status |
sp. nov. |
Cebrennus rechenbergi View in CoL spec. nov.
Figs 109–127 View FIGURES 109 – 117 View FIGURES 118 – 127 , 135–146 View FIGURES 135 – 140 View FIGURES 141 – 146 , 149–161 View FIGURES 147 – 151 View FIGURES 152 – 161 , 173 View FIGURE 173
Cebrennus villosus (Jézéquel and Junqua) View in CoL . King 2013: 7 ff., figs 2.1–2.8, 4.1 (illustration of flic-flac behaviour; misidentification)
Type material. MOROCCO: Meknès-Tafilalet: Holotype male (PJ 3187), Er Rachidia, 30 km SE Erfoud, near Tisserdmine, northern Erg Chebbi, 31° 16.212' N 3° 59.475' W, 760 m elev., sand dunes, I. Rechenberg leg. 15 July 2009, SD 622 ( SMF 59794). Paratypes: 1 male (PJ 3395), leg. 2008 ( SMF); 1 male (PJ 3407), leg. 14 August 2010, SD 392 ( SMF); 1 male (PJ 3189) leg. 27 July 2009 ( SMF 59796); 1 female (PJ 3186) leg. 14 July 2009 ( SMF 59793); 1 female (PJ 3188) leg. 25 July 2009 ( SMF 59795), 1 male (PJ 3477, SD 435), 1 female (PJ 3478, SD 439), leg. 1 September 2013 ( ZMB); 1 male (PJ 3479, SD 463), 1 female (PJ 3480), leg. 1 September 2013 ( SMF); 1 male (PJ 3481, SD 479), 1 female (PJ 3482, SD 480), leg. 1 September 2013 ( SMF), all other data as for holotype.
Additional material examined. 3 juveniles, as for holotype except for: leg. 5. July 2009 ( SMF 59797).
Diagnosis. Medium-sized Sparassinae, body length of males: 13.8–19.0, females: 19.0–19.5. Similar to C. villosus and C. concolor in having the distal embolus coiled, the RTA reduced ( Figs 109–114 View FIGURES 109 – 117 ) and epigyne with a simple and long median septum ( Figs 118, 123 View FIGURES 118 – 127 ). It is distinguished from C. concolor (only males known) by the blunt distal tip of cymbium, the absence of a gap between the strong rounded part of embolus and the tegulum and the slightly smaller RTA, that is not reaching the distal tibial margin in retrolateral view. Males of the new species can be distinguished from those of C. villosus by the presence of a small RTA. Females are hard to distinguish from those of the very similar C. villosus . Both species exhibits small pits at the lateral epigyne originated from deeply invaginated muscular attachment points. These are slightly deeper and more pronounced in C. rechenbergi spec. nov. Females exhibit a small, but distinct transversal ridge close to the posterior end of the medium septum (it may be functionally connected with anchoring the male RTA considering functional morphology of other Sparassidae ; Jäger unpubl. data). The rounded median structures of the internal duct system (glandular appendages) are relatively larger than in C. villosus ( Figs 119, 124 View FIGURES 118 – 127 ), i.e. their largest diameter as large or larger than the distance from these structure to the anterior end of the internal duct system (generally smaller in C. villosus ), and by the septum relatively longer to the length of the internal duct system (septum length/length internal duct system = 0.69–0.72; C. villosus : 0.57–0.66). Moreover, the two posterior teeth are in both sexes more distinctly fused ( Figs 116 View FIGURES 109 – 117 , 121, 126 View FIGURES 118 – 127 ) than in C. villosus , i.e. the apices are relatively shorter than those in C. villosus . The flic-flac (escape) behaviour of C. rechenbergi spec. nov. ( Figs 152–161 View FIGURES 152 – 161 ) seems also to be unique and was not observed in C. villosus so far.
Etymology. The specific name is honouring Prof. Dr. Ingo Rechenberg, who collected the type material. Moreover, he spent many months in the Saharan desert observing and filming the unusual behaviour of this new species and triggered research in the field of bionics and bio-robotics; noun (name) in genitive case.
Description. Male (holotype first with paratypes in parentheses if differing). PL 8.2 (7.2–8.5), PW 6.5 (5.8–6.7), AW 3.9 (3.3–3.8), OL 8.7 (6.6–10.5), OW 7.0 (4.9–6.3). AME 0.62 (0.57–0.70), ALE 0.39 (0.35–0.38), PME 0.35 (0.36–0.40), PLE 0.37 (0.31–0.41), AME–AME 0.10 (0.15), AME–ALE 0.06 (0.08–0.10), PME–PME 0.54 (0.56–0.61), PME–PLE 0.61 (0.55–0.66), AME–PME 0.41 (0.45–0.48), ALE–PLE 0.46 (0.52-0.53), clypeus AME 0.17 (0.20), clypeus ALE 0.32 (0.28–0.30) ( Fig. 117 View FIGURES 109 – 117 ). Spination: Palp: 120 (020), 0 0 0, 0 0 0, 000; legs: femur I–III 323, IV 322(3); patella 000; tibia I–IV 2024; metatarsus I–III 2024, IV 3036 (3035/4024). Ventral metatarsi I–IV with sparse scopula in distal half, without spines or bristles distally. Leg formula: 2413. Measurements of palp and legs: Palp 10.8 (3.8, 1.6, 1.7, -, 3.7), leg I 37.8 (11.5, 3.6, 9.8, 10.3, 2.6), leg II 40.9 (12.2, 4.0, 10.6, 11.5, 2.6), leg III 32.6 (10.3, 3.5, 8.0, 8.6, 2.2), leg IV 37.9 (12.1, 3.7, 9.3, 10.3, 2.5). Cheliceral furrow with 2 anterior and 2 adnate posterior teeth, without denticles ( Fig. 116 View FIGURES 109 – 117 ). Margin of chelicerae close to fang base with 6/9 (7–9) bristles.
Palp as in diagnosis ( Figs 109–114 View FIGURES 109 – 117 ). Tibia distinctly shorter than cymbium, short RTA with concave distal margin, disto-retrolaterad. Embolus arising in a 9-o’clock-position, distal coil situated in a 1- to 3-o’clock-position; embolus tip reaching into retrolateral half of that coil. Subtegulum visible at distal and prolateral margin of alveolus.
Colouration in ethanol: Pale yellowish-brown without distinct pattern. Dorsal prosoma with distinct longitudinal fovea and slight radial pattern. Ventral side lighter; distal segments of appendages slightly darker; spinnerets appearing in ventral view dark by dark setae, otherwise body and legs with white setae; tips of chelicerae, fangs, and distal leg claw tufts dark. Live specimens exhibiting yellow areas on dorsal opisthosoma and femora, otherwise the spider appears white shimmering with ventral leg scopulae in black ( Figs 141–144 View FIGURES 141 – 146 ).
Female (2 paratypes, PJ 3186, 3188): PL 7.3–8.2, PW 5.8–6.3, AW 4.0–4.4, OL 9.0–11.3, OW 6.5–8.6. AME 0.62–0.67, ALE 0.38–0.40, PME 0.38–0.40, PLE 0.35–0.41, AME–AME 0.16–0.20, AME–ALE 0.0.06–0.09, PME–PME 0.65, PME–PLE 0.57–0.72, AME–PME 0.47, ALE–PLE 0.51–0.59, clypeus AME 0.26–0.27, clypeus ALE 0.31–0.33 ( Fig. 122, 127 View FIGURES 118 – 127 ). Spination: Palp: 120, 0 0 0, 0 0 0 0, 1000; legs: femur I–II 323, III 32 (1)3, IV 322; patella 000; tibia I–III 2024, IV 2024 (3); metatarsus I–II 2024, III 2024 (2014, 1024), IV 3035. Ventral metatarsi I–IV with sparse scopula in distal half, without spines or bristles distally. Leg formula: 2413(4213). Measurements of palp and legs: Palp 10.4–11.0 (3.5–3.6, 1.5–1.6, 1.9–2.2, -, 3.5–3.6), leg I 30.2–32.2 (9.1–9.7, 3.5–3.7, 7.2–7.8, 8.1–8.5, 2.3–2.5), leg II 31.7–33.0.8 (9.7–10.2, 3.6–3.7, 7.7–8.4, 8.3–9.0, 2.4–2.5), leg III 25.7–26.6 (8.0–8.5, 3.2–3.3, 5.8–5.9, 6.5–6.8, 2.1–2.2), leg IV 31.9–32.7 (10.1–10.6, 3.5, 7.2–7.9, 7.9–8.3, 2.2–2.4). Cheliceral furrow with 2 anterior and 2 adnate posterior teeth, without denticles ( Fig. 121, 126 View FIGURES 118 – 127 ). Margin of chelicerae close to fang base with 4–12 bristles. Palpal claw with ca. 7–9 teeth.
Copulatory organ as in diagnosis ( Figs 118–120, 123–125 View FIGURES 118 – 127 ). Epigynal field only well distinguishable in the posterior half, with one slit sensillum on each side. Internal duct system with anteriorly situated membranous sacs (atria) of diverse shapes; medially with slightly sclerotized oval structures originated from a membranous zone. Fertilisation ducts flattened, their tips bent at a right angle.
Colouration in ethanol: As in male. For live colouration see Figs 135–139 View FIGURES 135 – 140 , 141–144 View FIGURES 141 – 146 .
Distribution. Only known from the type locality ( Fig. 173 View FIGURE 173 ).
Biology and behaviour. The following data are compiled mainly from field observations and shots by Ingo Rechenberg in Morocco. Parts of these observations have been used and published by King (2013).
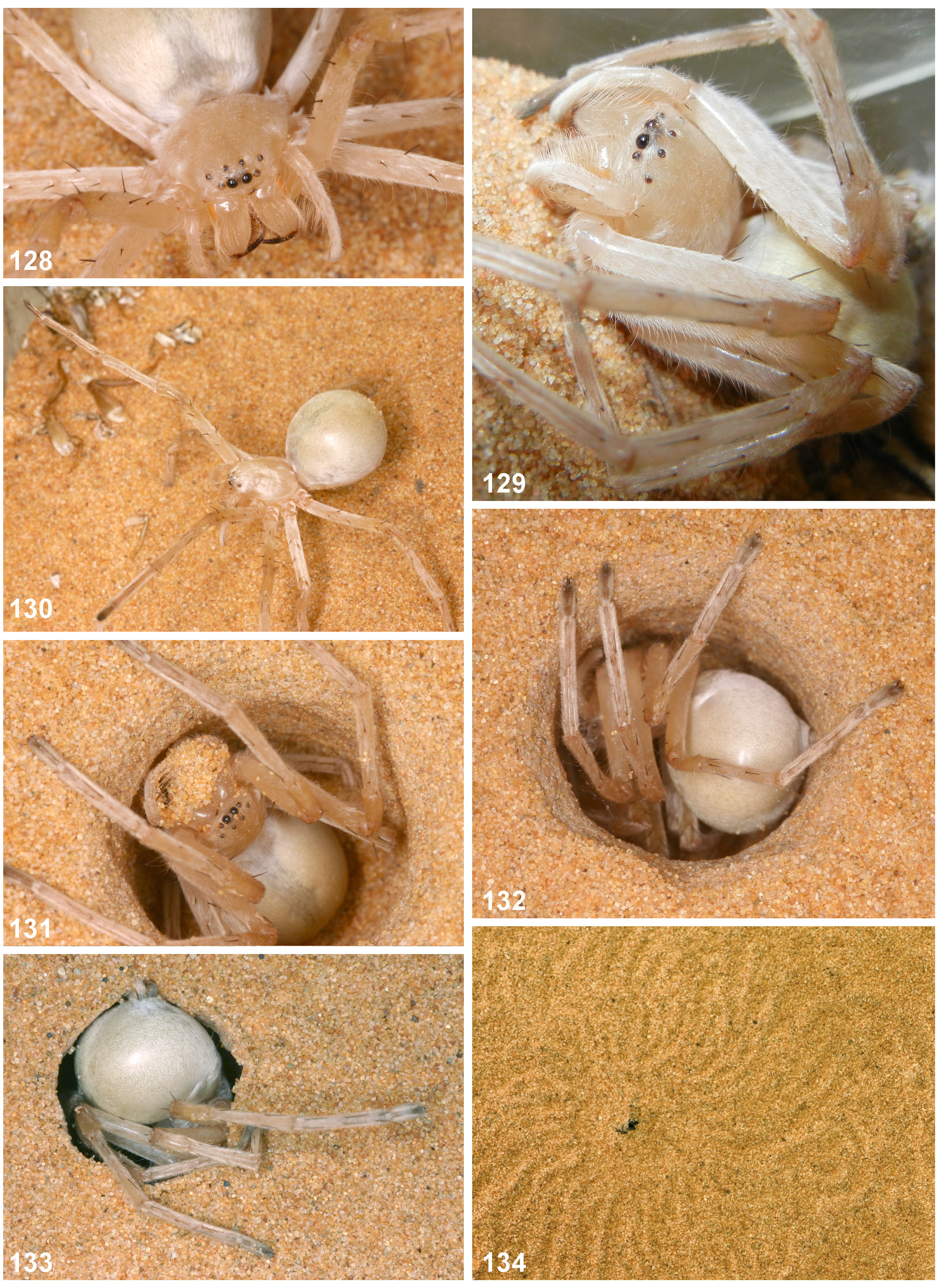
C. rechenbergi spec. nov. lives in sandy deserts in the Erg Chebbi ( Fig. 173 View FIGURE 173 ) and builds similar vertical burrows ( Figs 149–151 View FIGURES 147 – 151 ) like C. villosus (compare “Biology” paragraph under C. villosus ). Although no direct observations were made the same array of long bristles at palps and chelicerae let assume that spiders carry the loose sand in the same way as C. villosus ( Fig. 131 View FIGURES 128 – 134 ). Some of the tubes sticking out of the sand in the morning exhibited an elongation part ( Figs. 149, 150 View FIGURES 147 – 151 right). It is not clear yet how the spiders construct this part, but it is considerably narrower than the rest. Probably it can be interpreted as energy saving issue in a habitat with limited prey items. Further it was observed that the spider returns after its nocturnal trip to its burrow and “dives” through a slit of the lid ( Figs 135–140 View FIGURES 135 – 140 ) starting with leg II and III of one side (here right side, Fig. 136 View FIGURES 135 – 140 ), then first disappearing with one side of the body and continuing with the rest.
Copulatory posture is the same as in other Sparassidae ( Micrommata virescens , Heteropoda venatoria , Heteropoda tetrica , Holconia insignis ; Jäger unpubl.): the male is sitting on the back of the female in a reverse position, i.e. with his head at her opisthosoma, no matter whether the female is sitting on the desert ground or hanging at grass leafs ( Figs 141–144 View FIGURES 141 – 146 ). Palps are inserted alternatively: left palp at the left side of the female and right palp accordingly. From a close-up ( Fig. 144 View FIGURES 141 – 146 ) it is clear that the RTA comes close to the epigynal pits as well as to the transversal ridge of the medium septum during the copulation process, theoretically supporting the hypothesis that these structure build a functional unit.
The most striking behaviour is the so-called flic-flac behaviour: when disturbed a spider will usually show first the threatening behaviour like C. villosus ( Fig. 145 View FIGURES 141 – 146 ; compare for C. villosus Jäger 2000 and Fig. 130 View FIGURES 128 – 134 ). When further provoked the spider starts running and in about 50% of all cases observed running transforms to flic-flac behaviour. Usually it is performed forwards ( Figs. 152–161 View FIGURES 152 – 161 ), but can also be done backwards. It can be performed on even ground but also down- or up-hill ( Fig. 161 View FIGURES 152 – 161 ). It could be interpreted as last-resort escaping behaviour. Speed is two times higher than running speed (according to high speed camera shots and subsequent analysis by I. Rechenberg) and might be the trigger to select for this costly behaviour. However, I. Rechenberg observed that males and females perform this behaviour and that the spider will approach the person who provoked it. Spiders got to the hand, the knee or to other body parts of that person, in one case a spider choose the torch lying in the sand beside the person. Such behaviour is not known from any of the other Cebrennus species, but in the Kalahari(?) desert photo evidence exists from a Sparassidae ( Leucorchestris ? sp.) jumping on the back from a suricate (Henschel, personal communication). Mock attacks were also observed in Namibia by Henschel on a dune lark (which fled after the attack) and by Norgaard on himself on several occasions (both unpublished observations). It may be interpreted as best option to defend, since running away is useless in a habitat without any possibilities to hide and with temperatures making a spider exhausted after several metres running (Norgaard, personal communication).
The movement itself is induced mainly by legs I, II and IV and their quick and powerful stretching. The spider will jump, tumble and land on the dorsal tarsi and metatarsi IV ( Fig. 156 View FIGURES 152 – 161 ). Subsequently, tarsi II are in contact with the ground. In this stage legs I and III are held above the body ( Fig. 157 View FIGURES 152 – 161 ). After that, dorsal tarsi and metatarsi I touch the sand ( Fig. 158 View FIGURES 152 – 161 ). At least tarsi II and IV dive slightly into the sand, when oriented in a right angle to the sand’s surface ( Fig. 158 View FIGURES 152 – 161 ). The flic-flac behaviour is not known from C. villosus ( Algeria, Tunisia) or C. concolor ( Egypt) , two closely related species according to their palpal morphology.
Similar behaviours are known from the huntsman spiders Carparachne aureoflava Lawrence, 1966 and C. alba Lawrence, 1962 (gravity-driven wheeling in Namib dunes; Henschel 1990). Another wheeling behaviour is known from larvae of the tiger beetle Cicindela dorsalis media (LeConte, 1856) (wind-powered somersaults in Namib; Harvey & Zukoff 2011) and from an unidentified Salticidae (wind-powered wheeling in Namib; Henschel 1990). Caterpillars of two species ( Brackenbury 1997, 1999) as well as a mantis shrimp ( Full et al. 1993) perform active backward somersault and subsequent wheeling. Gravity-driven rolling is known from Glomeridae, Sphaerotheriida (pill millipedes, giant pill millipedes; Myriapoda) and Armadillidiidae (pill bugs; Crustacea). In all cases it is reported that speed is increased when the particular behaviour is applied. In two examples it seems to be an evolutionary response to parasitic wasps that hunt the tiger beetle larvae or the golden wheel spider, in others it is when animals are provoked, disturbed or threatened. In C. rechenbergi spec. nov. the natural triggering factor is yet unknown. In contrast to the wheeling behaviour in Carparachne aureoflava it is an active energy-consuming movement, which can lead to the death of the individual when performed some times in succession (Rechenberg, personal communication). The unique flic-flac behaviour of C. rechenbergi spec. nov. inspired King (2013) to investigate this form of locomotion structurally and to invent a robot with similar motional elements.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cebrennus rechenbergi
| Jäger, Peter 2014 |
Cebrennus villosus (Jézéquel and Junqua)
| King 2013: 7 |